Reaxys Predictive Retrosynthesis powered by Iktos release notes for April 2025
Last updated on April 02, 2025
This release focuses on the user experience by introducing the option to ignore stereochemistry and automating the re-launch of jobs with extended parameters, reducing instances of no results found.
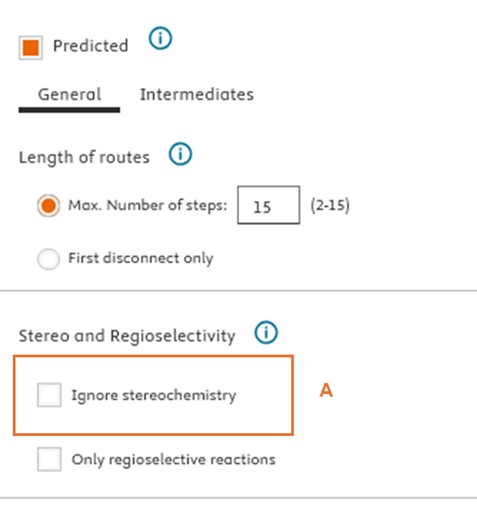
Ignore stereochemistry feature
Reaxys Predictive Retrosynthesis powered by Iktos offers the capability to predict synthesis routes for compounds with defined stereochemistry using a “chiral pool” approach. This method identifies routes where stereocenters are derived from building blocks with the desired stereoconfiguration, rather than being formed during the synthesis process. However, we noticed that sometimes users wanted to ignore the stereochemistry of compounds while synthesis route planning.
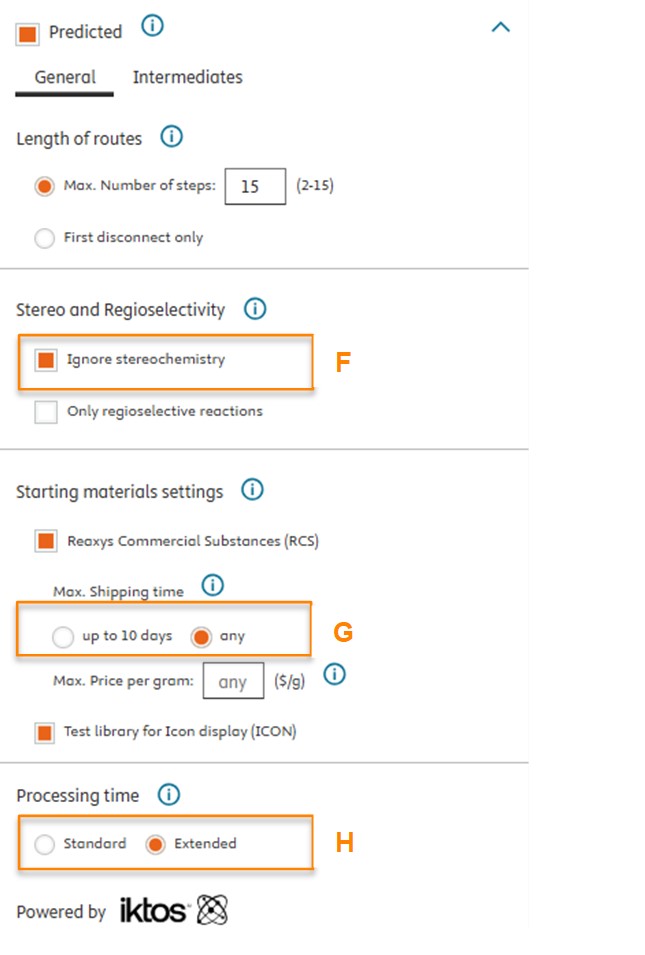
We have introduced an “Ignore Stereochemistry” checkbox (A) for users who wish to bypass molecular stereochemistry considerations. When selected, this option allows Reaxys Predictive Retrosynthesis to search for routes without regard to the stereochemistry of intermediates and building blocks. Please note that despite the predicted intermediate and building blocks in the route will be shown achiral (B), users will still see the target compound with specified stereoinformation (C), which however is ignored in synthesis route planning.
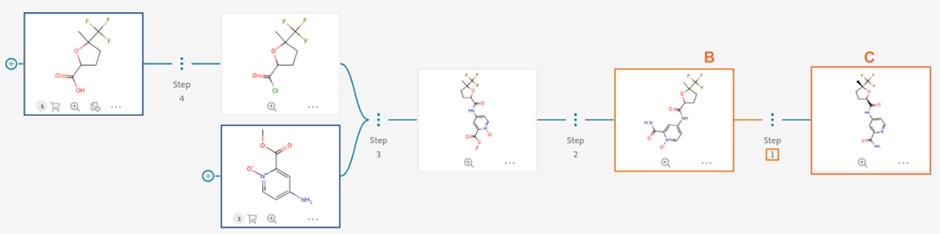
It is expected to return more predicted routes when “Ignore stereochemistry” is enabled. This feature has no effect on achiral compounds.
Automation of prediction processing parameters in case of no predicted results
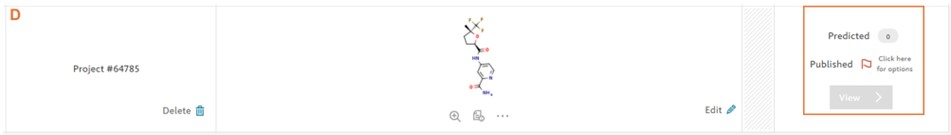
Reaxys Predictive Retrosynthesis produces predicted synthesis routes for small drug like molecules in more than 90% of the cases. However, in some cases e.g. complex heterocycles, molecules with multiple fused ring systems, complex natural products, macrocyclic molecules, it may happen that the predictive retrosynthesis model is unable to resolve the synthesis pathway back to commercially available starting materials (D).
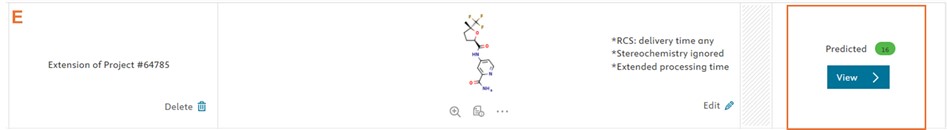
Currently, when this happens, users are advised to edit the default parameters and re-run the prediction. To improve user experience, this manual step has now been automated, and it increases the probability of predictive retrosynthesis model providing a synthesis route (E).
Reaxys predictive retrosynthesis powered by Iktos automation of no results
In the event of no results, the default settings are automatically changed to extended parameters to increase the likelihood of obtaining a predicted synthesis route. In summary, the extended parameters will be modified as follows:
- The stereochemistry defined in the target molecule is ignored (F)
- The building block library is changed to “RCS any” which include more building blocks (G)
- The processing time is changed to Extended (H)
In case the extended parameters fail to predict a synthesis route, then a ‘First step disconnection’ is automatically run giving the users some ideas about where they could potentially start breaking the target molecule.
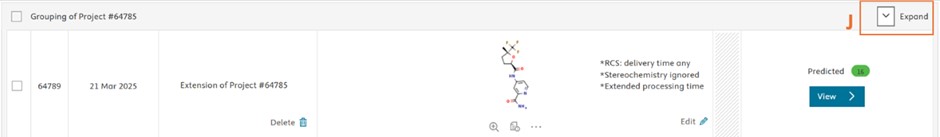
The results of the original and extended projects are grouped for easier navigation. All projects within a group can be made visible by clicking “Expand” button (J).
Did we answer your question?
Related answers
Recently viewed answers
Functionality disabled due to your cookie preferences