Clinical Trials FAQs
Last updated on May 05, 2025
- What are clinical trials?
Clinical trials are systematic investigations that assess the safety and efficacy of new medical interventions, including drugs, therapies, and devices. Conducted with human participants, these studies generate vital data that informs clinical practice and therapeutic guidelines. In the context of Embase, clinical trials provide valuable insights into the latest research developments and treatment options available in the biomedical field. They are essential for researchers and healthcare professionals seeking to stay informed about emerging therapies and evidence-based practices. - What is ClinicalTrials.gov?
ClinicalTrials.gov is a comprehensive online database provided by the U.S. National Library of Medicine. It serves as a registry and results database for publicly and privately supported clinical studies conducted around the world. The database includes information on a wide range of clinical trials, including those investigating new drugs, therapies, medical devices, and treatment approaches across different medical conditions and patient populations. - What new content will be available in Embase from ClinicalTrials.gov?
Embase will soon include clinical trials obtained solely from ClinicalTrials.gov, significantly broadening the range of research materials to encompass more than 500,000 trials currently available in the database. How are you adding this data?
We will be adding approximately 20,000 clinical trials per day to Embase.com. This means around 100,000 new clinical trials will be added each week. This process will take up to 6 weeks to complete.We will start loading data on 5th May 2025 and by 16th June 2025, all trials from clinicaltrials.gov will be on Embase.
- How will clinical trial records be identified in Embase?
On the Results page, all clinical trials will be marked with a "CLINICAL TRIAL" label, making it easy for users to identify this type of content.
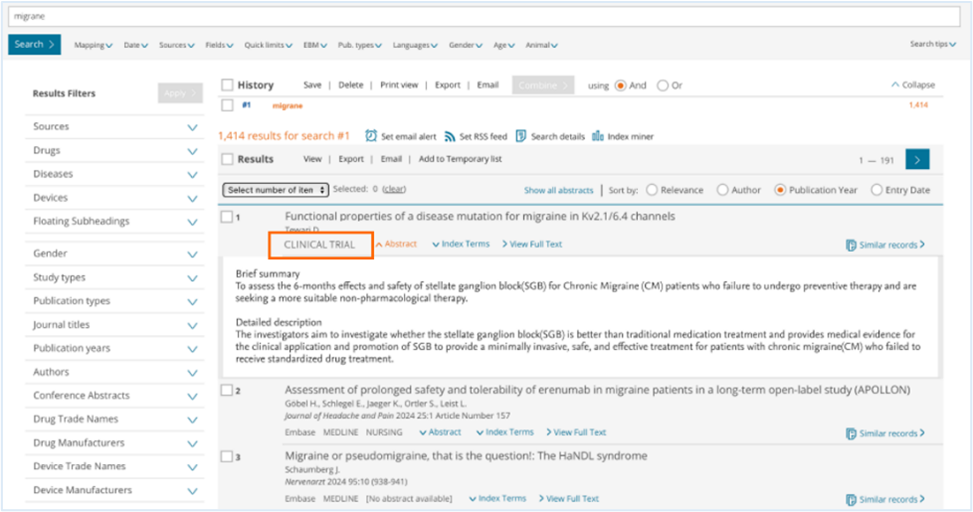
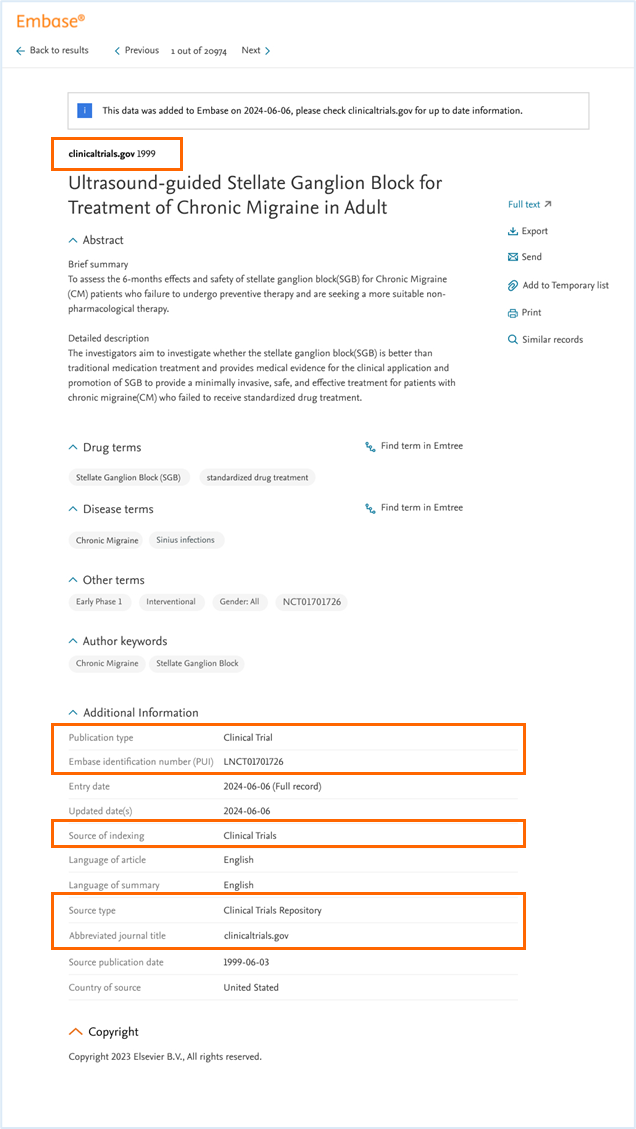
On the Record Details Page, there will be several new values under ‘Additional information’, as explained in the image below.
- How can I limit or exclude clinical trials from my search results based on queries?
- To exclude clinical trials from your search results, add the following to your query: NOT 'clinical trial'/it
- To search exclusively for clinical trials, use: AND 'clinical trial'/it
- How can I limit or exclude clinical trials from my search results using UI filters?
Embase will soon include clinical trials obtained solely from ClinicalTrials.gov, significantly broadening the range of research materials to encompass more than 500,000 trials currently available in the database.Results filters below the search bar
Sources – Limit your content selection by using the new ‘Clinical trials’ option.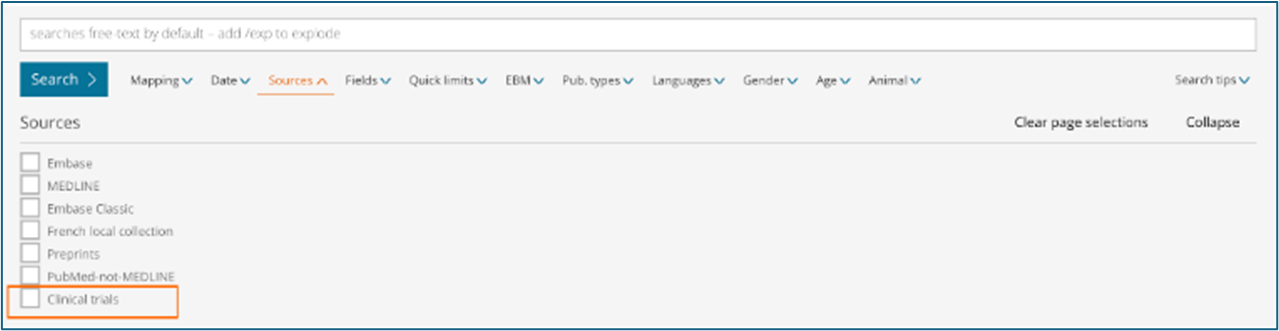
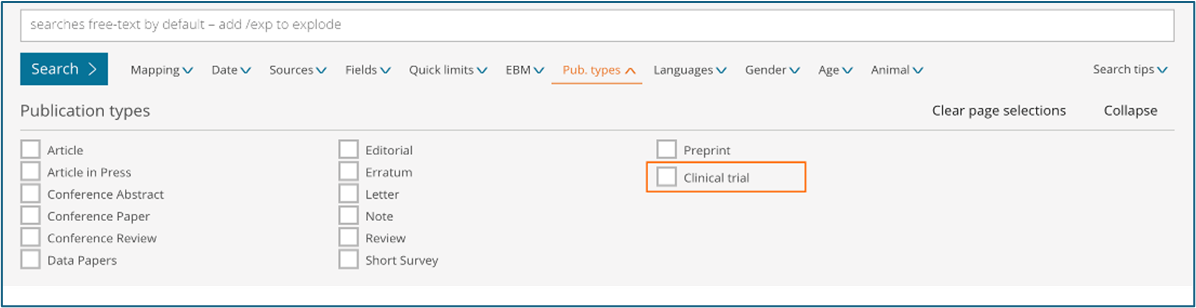
Publication type – Limit your content selection by using the new ‘Clinical trial’ option
Results filters on the left side of the page
Sources - Limit your content selection by using the new ‘Clinical trials’ option
Publication types– Limit your content selection by using the new ‘Clinical trial’ option
Journal titles– Limit your content selection by using the new ‘clinicaltrials.gov’ option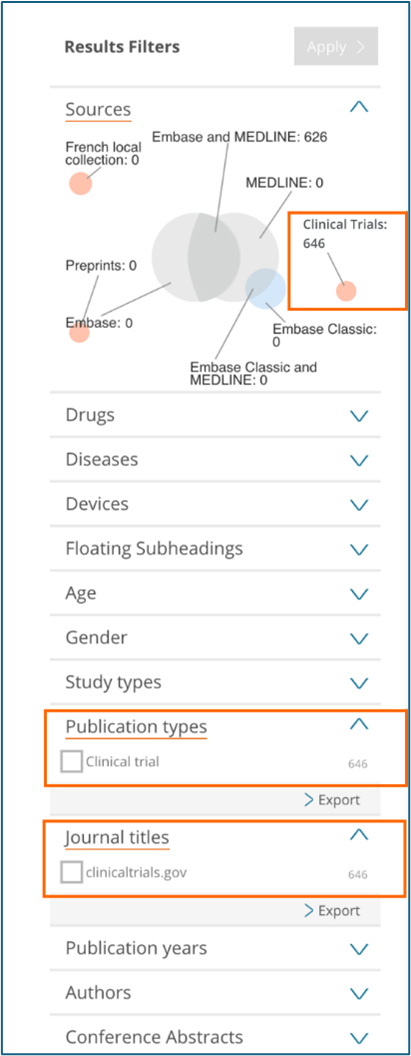
What dates are shown for clinical trials?
Clinical trial records will include the following dates in the Additional Information section:
- Entry date (the date when the trial record was added to Embase)
- Update date (marking any updates made to the record after it was added to Embase)
- Source publication date (First submitted date on clinicaltrials.gov).
- Will clinical trials be included in my existing saved searches?
Yes, running existing saved searches after the release of clinical trials will generate more results, as they will now include clinical trials along with other content types. If you do not wish to receive clinical trials content, we recommend editing your saved searches to include the exclusion query: NOT 'clinical trial'/it - Will clinical trials be included in email alerts?
At this stage, clinical trials will not be included in email alerts. However, we plan to add functionality in the future that will allow users to include or exclude clinical trials from their email alerts. - How will clinical trials be indexed in Embase?
Clinical trials were automatically indexed using the latest Emtree vocabulary at the time (Emtree 2024.3), ensuring that they were indexed accurately based on their title, brief summary, and detailed description. - How are NCT numbers and PUI linked?
PUI numbers for Clinical trial records will be their respective NCT numbers prefixed by ‘L’, for example – LNCT12345678. - How can we use NCT number to search a particular clinical trial record?
Using the NCT number will fetch all the Embase records having that NCT number mentioned anywhere in the indexed text – this will include all content types. However, to search for a particular clinical trial record using its respective NCT number, prefix the NCT number with ‘L’ and search using this as its PUI number. This will fetch that particular CT record only. - How long will it take to index a clinical trial record?
All clinical trial records will be fully indexed (automatically) before they are published on Embase. - Will there be regular updates to the clinical trials data?
The first release will be a one-time data load of all trials from clinicaltrials.gov. In the future, we plan to have regular content updates, and we have already started working on this feature. - Is clinical trial data available in Medline/Pubmed?
Pubmed/Medline only provide related published content (like journals) having any reference of the clinical trials. They do not provide content from clinicaltrials.gov. - What CT sponsor information is available in an Embase record?
Sponsor Information is not in the scope of the release in May 2025. We are considering adding sponsor information in the future. - How does the addition of clinical trials affect PV workflows?
Clinical trials will not be included in the alerts for this version. However, the searches will have additional records from CT content and can be filtered out if not required from PV workflows. - Are we cross linking a clinical trials with publications?
Users will be able to find all Embase records that are referring to a clinical trial, by searching for that trial NCT number. - Does clinicaltrials.gov have a datasets option from the site itself?
Yes - https://clinicaltrials.gov/data-api/api#extapi - Is monitoring clinicaltrials.gov mandatory for clinical evaluation of medical devices?It is advisable to visit clinicaltrials.gov for the latest developments & updates for medical devices.
- How will clinical trials be returned in API queries?
API queries will also return clinical trials by default, just like they will be included in the Embase.com search. Users can exclude clinical trials from API results by adding the query: NOT 'clinical trial'/it - Where can I find more information about this new content addition?
For additional details or specific inquiries, please feel free to reach out to our support team or visit the Embase website for the latest updates. You can read more about this release here: https://service.elsevier.com/app/release_notes/supporthub/embase/#Mar25
Did we answer your question?
Related answers
Recently viewed answers
Functionality disabled due to your cookie preferences