Embase Release Notes
Last updated on February 12, 2026
Please find below the latest Embase release notes:
More clinical trial data available on Record Details Page
We are excited to announce a major update to the Clinical Trial record details page in Embase. This redesign is based on your feedback and aims to make trial information more comprehensive, easier to scan, and faster to access.
What’s new?
Enhanced header section
In addition to the official title, you can now access key information like trial status, tag indicating whether results are available, the most recent update date from clinicaltrials.gov in the header section.

Study Overview
The abstract section has been renamed to Study Overview. It includes both a Breif Sumary and Detailed Description.
- Added more information about the study (new sections added)
Collaborators and investigators
This section provides information about who is responsible for the study, including the main sponsor, principal investigator, and any collaborating organizations.

Study locations
This section lists the full name and address of each site where the clinical study is being conducted

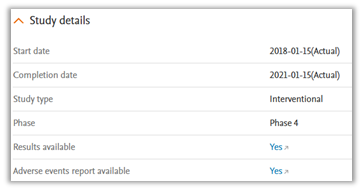
Study Details
This section provides key information about the trial, including when it started and finished, the type of study, its phase, and whether results or adverse events have been reported. Help you to understand the availability of outcome data, with links to results when available.
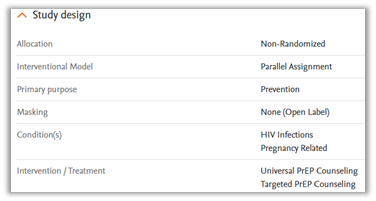
Study design
This section provides information on the trial structure, the way participants are assigned to groups, the intervention strategy, and the main goal of the study. It also includes details on masking, the conditions being studied, and the treatments or interventions involved, such as drugs, devices, etc.
Participation criteria
This section provides list of criteria for selection of participants in clinical study. It includes information about enrollment, Gender, Minimum age, Maximum age, Healthy volunteers, Inclusion criteria and Exclusion criteria.

Find Linked articles
A new option is added on the right-hand panel; it will run a search to retrieve the related articles referencing this trial. This feature will enable you to easily discover related publications and research in one place.
What stays the same?
- Export – Export functionality remains unchanged. The new, additional trial details are not included in the exported files yet. In the future, you will be able to export this additional data in CSV and RIS formats.
- Send – Email content remains unchanged, additional trial details are not included in the email yet.
- Add to Temporary List – You can add the title, abstract, and index terms to your temporary list. However, the expanded trial data cannot be included in the list yet.
- API customers – No change for API customers in this release. In the future, enhanced CT content will also be delivered via API.
- Flat file customers - No change for Flat file customer in this release. In future, enhanced CT content will also be delivered via the Flat files.
- Email alerts – Clinical trials are not included in the alerts in this release.
Added retraction indicators for tombstone (retracted) records
For the tombstone (retracted) records, we have added a prominent retraction banner at the top of the Record Details page and the Cite button has been disabled for these items.

This update will help you to quickly identify the retracted records, and it reduces the risk of citing retracted work. This change is only applicable to the tombstone (retracted) records.
Fixed bugs
- Resolved issues affecting the CITE button functionality
- Accessibility improvement in alignment with WCAG 2.1 AA standards
As part of our commitment to provide comprehensive coverage, we will be actively backfilling clinical trial data from the May update through to September.
Embase will ingest around 65,000 clinical trial records from ClinicalTrials.gov to complete the backfill from the May release.
These additional clinical trial records will be added between 13th October to 16th October 2025. We will be adding 20,000 trials per day and the process will complete by 16th October.
During this period, you may notice an increase in the number of search results as the new content becomes available.
Embase.com
During this period, you may notice an increase in the number of search results as the new content becomes available.
To exclude clinical trials from your search results or saved searches or saved list, add any one of the following conditions to your search query:
- NOT 'clinical trial'/it (this excludes based on publication type)
- NOT 'clinical trial':dtype (this excludes based on database collection)
- NOT 'clinicaltrials.gov'/jt (this excludes based on the journal title "clinicaltrials.gov")
The clinical trials will not be included in email alerts.
API
When this data load takes place, the API queries will return clinical trials by default, in the same way they are included in the embase.com search results.
To exclude clinical trials from your API results, add any one of the following conditions to your search query:
- NOT 'clinical trial'/it (this excludes based on publication type)
- NOT 'clinical trial':dtype (this excludes based on database collection)
- NOT 'clinicaltrials.gov'/jt (this excludes based on the journal title "clinicaltrials.gov")
If you do not apply filters to exclude this additional content, you will notice an increase in the number of search results you receive.
Flat files
Following the data load, the update folders will begin to include this additional content, and the subsequent monthly bulk file will also contain the full Clinical trial backfill.
Users can determine if a Clinical Trial record in the XML output is new or updated by checking the state attribute within the <ait:status> element.
For new clinical trial, the state attribute will include new
<ait:status stage="S300" state="new" type="core"/>
For updated clinical trial, the state attribute will include update
<ait:status stage="S300" state="update" type="core"/>
We have launched a new version of Embase AI. This new version contains the following:
Changes in the LLM functionalities
The team developed several new features and bug fixes and ran focused evaluation to test them. Highlights include:
- Multi-language support (tested with Japanese, Chinese (simplified), Korean, Italian, German, French, Spanish and Brazilian Portuguese)
- Use of tables in system responses when appropriate
- Improved follow-up prompt to avoid previous context bleeding into the rephrased follow-up question
- Improved retry prompts to avoid cases of zero results or syntax error
- Option to limit search to clinicaltrials.gov in query translation
- Improved detection of "results only" case where no natural-language summary should be produced
Moreover, the prompt and gold answers for the Comprehensiveness metric were completely rewritten to generate more meaningful evaluation results.
Changes in Embase AI functionality
- Summary will now include up to 10 references instead of 5.
- Ability to export the references used for the summary in different formats.
- Embase AI tab visible for non-registered users to increase its internal awareness.
- To increase its visibility, we have added a “New” sign near to the tab name.
Several bug fixed
As part of our continuing effort to expand content sources for your research, we will soon be adding Clinical Trials to Embase!
What are clinical trials?
Clinical trials are research studies that test the safety and effectiveness of new treatments, interventions, or medical devices. These trials are conducted to gather data and evidence to determine whether a new medical approach is safe, beneficial, and superior to existing treatments. Clinical trials play a crucial role in advancing medical knowledge, improving patient care, and developing new therapies for various health conditions.
What is clinicaltrials.gov?
ClinicalTrials.gov is a comprehensive online database provided by the U.S. National Library of Medicine, which serves as a registry and results database of publicly and privately supported clinical studies conducted around the world. The database contains information on a wide range of clinical trials, including those investigating new drugs, therapies, medical devices, and treatment approaches across different medical conditions and patient populations.
Researchers, healthcare professionals, patients, and the general public can access ClinicalTrials.gov to search for ongoing and completed clinical trials, review study details, eligibility criteria, and outcomes. The database serves as a valuable resource for individuals seeking information about participating in clinical trials, staying informed about the latest research developments, and contributing to the advancement of medical science.
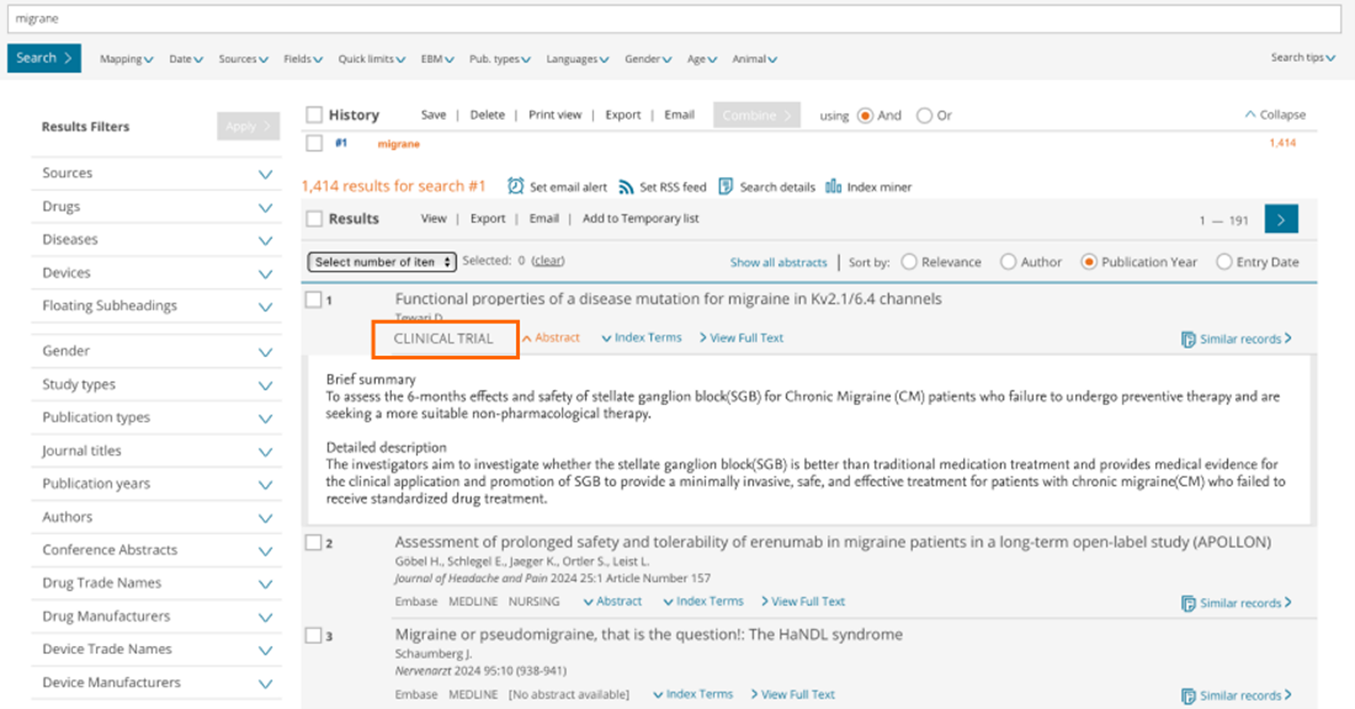
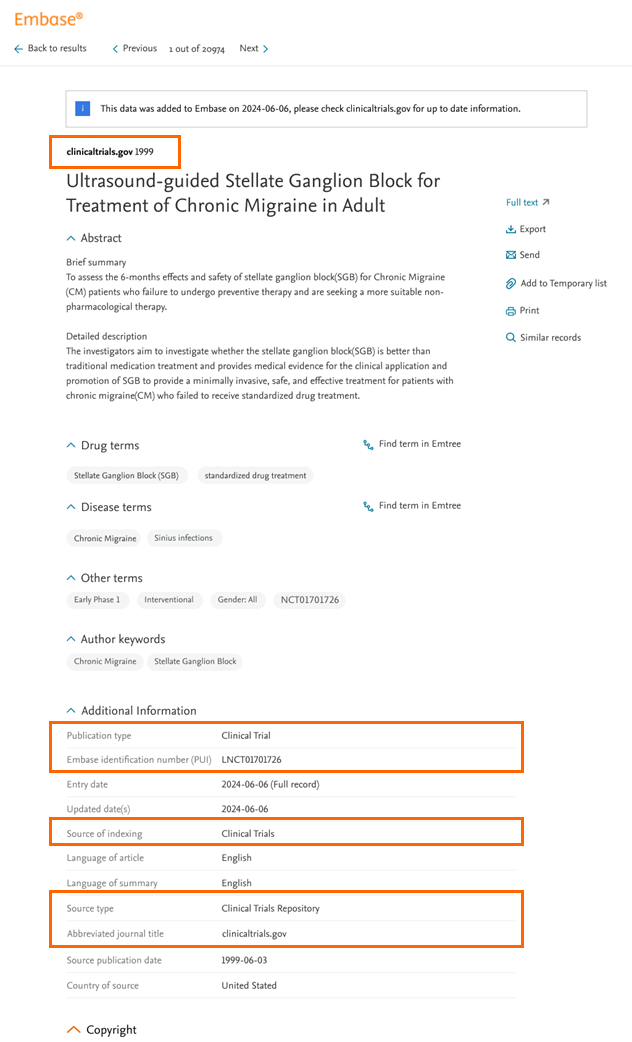
What will Clinical trials look like on Embase?
On the 'Results page', all Clinical trials will have a "CLINICAL TRIAL" label, so users can easily recognize this type of content.
On the 'Record Details Page', Clinical Trials records can be recognized in the additional information section, which includes:
- Publication Type: Clinical Trail
- Embase identification number (PUI): LNCT******** (this includes the original NCT number of that clinical trial)
- Source of indexing: Clinical Trials
- Source type: Clinical Trial Repository
- Abbreviated journal title: clinicaltrials.gov
How to access and how to limit clinical trials
Clinical trials will be added to the Embase general collection and will be available to all users at no additional cost.
When you search on Embase.com, you will now see a broader range of search results. This includes clinical trials alongside other content types such as articles, preprints, and conference abstracts.
If users wish to exclude clinical trials from their searches, they have to use query syntax as explained below.
New Clinical Trials Filters Available
Results filters below the search bar
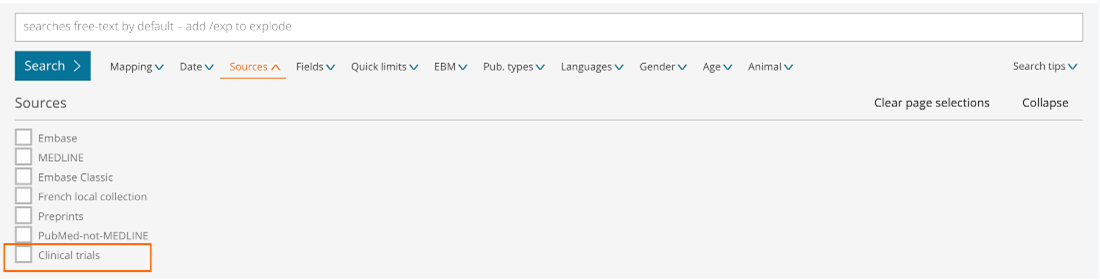
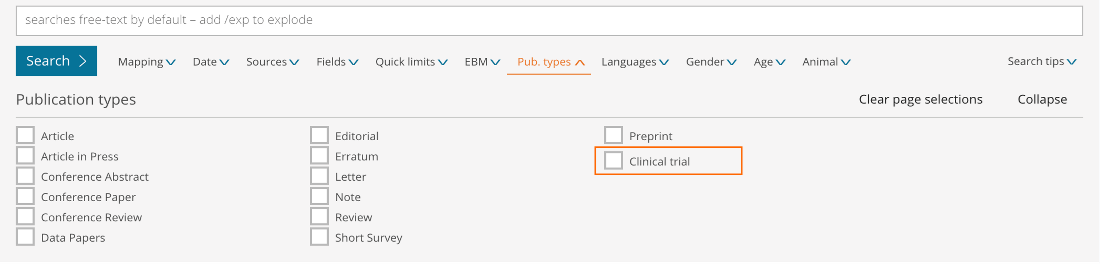
- Sources – Limit your content selection to only clinical trials by ticking the new ‘Clinical trials’ database collection option
- Publication type – Limit your content selection to only clinical trials by ticking the new ‘Clinical trial’ option
Results filters on the left side of the page
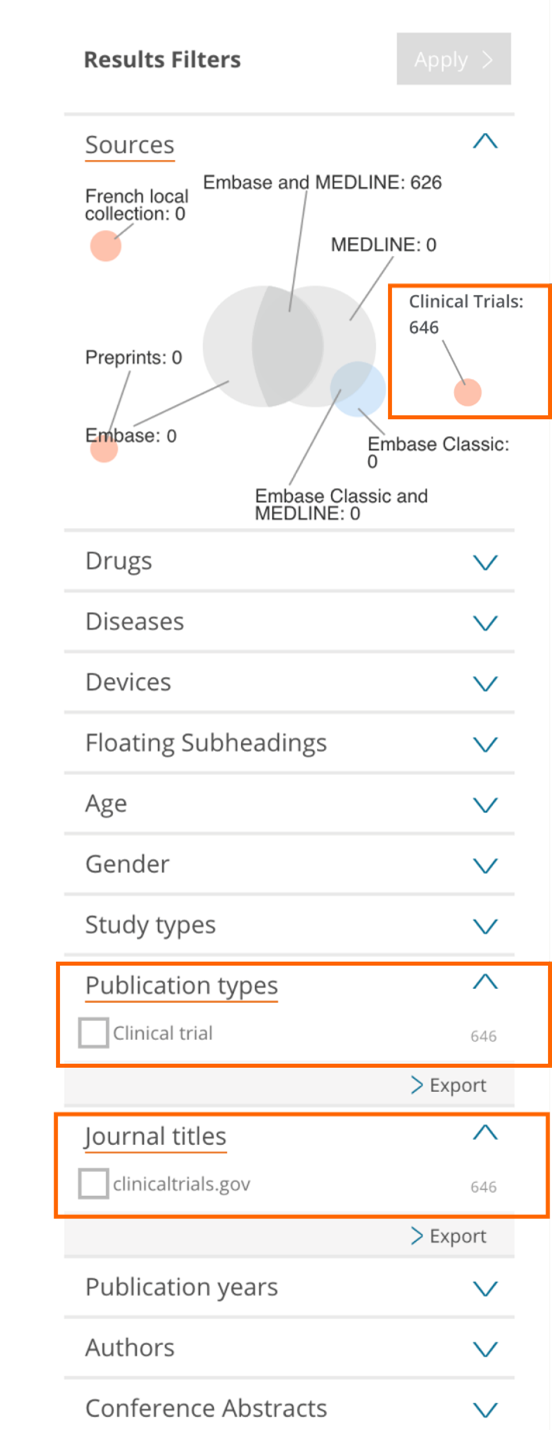
- Sources – Limit your content selection to only clinical trials by using the new ‘Clinical trials’ database collection option
- Publication types – Limit your content selection by using the new ‘Clinical trial’ option
- Journal titles – Limit your content selection by using the new ‘clinicaltrials.gov’ option
Using the query syntax to include or exclude clinical trials
Filter by Database Collection:
- Use NOT 'clinical trial':dtype to exclude all documents from the clinical trial database collection
Results may include other content like articles citing clinical trials or preprints relating to clinical trials, as long as these documents are NOT of the database collection type "clinical trial".
- Use AND 'clinical trial':dtype to get results that ONLY contain documents from the clinical trial database collection.
Filter by Publication Type:
- Use NOT 'clinical trial'/it to exclude all documents of the publication type "clinical trial".
Results may include other content like articles citing clinical trials or preprints relating to clinical trials, as long as these documents are NOT of the publication type "clinical trial".
- Use AND 'clinical trial'/it to get results that ONLY contain documents of the publication type "clinical trial".
Filter by Journal Title:
- Use NOT 'clinicaltrials.gov'/jt to exclude all documents under the journal title "clinicaltrials.gov".
- Use AND 'clinicaltrials.gov'/jt to get results that ONLY contain documents under the journal title "clinicaltrials.gov".
Indexing
Clinical trials will have the latest Emtree indexing (Emtree 2024.3). This will be automatic indexing based on the title, brief summary and detailed description.
Impact on users
We are excited to announce that we will be adding approximately 20,000 clinical trials per day to Embase.com. This means around 100,000 new clinical trials will be added each week. This process will take up to 6 weeks to complete. By 16th June 2025, all trials from clinicaltrials.gov will be on Embase.
During this time, if you do not apply filters to exclude this new content, you may notice an increase in the number of search results you receive.
Existing saved searches
Running saved searches after the release of Clinical trials will generate more results because results will include clinical trials along with the other content types (i.e. articles, preprints, conference abstracts, etc).
To exclude clinical trials from your saved searches, update to add one of the following filters to your search query:
- NOT 'clinical trial'/it (this excludes based on publication type)
- NOT 'clinical trial':dtype (this excludes based on database collection)
- NOT 'clinicaltrials.gov'/jt (this excludes based on the journal title "clinicaltrials.gov")
This will ensure clinical trials are not included in your search results.
New saved searches
Users will be able to create new saved searches which include or exclude clinical trials.
To exclude clinical trials from your new saved searches, add one of the following filters to your search query:
- NOT 'clinical trial'/it (this excludes based on publication type)
- NOT 'clinical trial':dtype (this excludes based on database collection)
- NOT 'clinicaltrials.gov'/jt (this excludes based on the journal title "clinicaltrials.gov")
To search only for clinical trials, add one of the following filters to your search query:
- AND 'clinical trial'/it
- AND 'clinical trial':dtype
- AND 'clinicaltrials.gov'/jt
Email alerts
Clinical trials will not be included in email alerts following this release. In the future, we plan to add the ability for the user to include or exclude clinical trials from their email alerts like we have done for preprints.
API
API queries will return clinical trials by default, in the same way they will be included in the embase.com search results.
To exclude clinical trials from your API results, add one of the following filters to your search query:
- NOT 'clinical trial'/it (this excludes based on publication type)
- NOT 'clinical trial':dtype (this excludes based on database collection)
- NOT 'clinicaltrials.gov'/jt (this excludes based on the journal title "clinicaltrials.gov")
Flat Files
Starting from 5th May 2025, the update folders will begin to include this new content, and the subsequent backfile will then contain the full CT backfile.
We will add approximately 20.000 clinical trials per day (i.e. 100.000 per week) over the course of around 6 weeks. By 16th June 2025, all trials from clinicaltrials.gov will be on Embase.
Important dates
Now: users can apply the new clinical trials limits and filters for their queries (e.g. 'clinical trial':dtype; 'clinical trial'/it; 'clinicaltrials.gov'/jt) to API queries
25th March 2025 - technical release that will result in filters for clinical trial content to appear on Embase.com - however, there will be no content associated yet with these filters
5th May 2025 - clinical trials content will start to be added
16th June 2025 - all trials from clinicaltrials.gov will be on Embase
Clinical Trials Document-Specific XML tags
We are implementing the following tags in the flat file XML output, to make it easier for users to identify this content.
These updates will appear only in the Clinical Trials XMLs located in both the daily update folder and the bulk DAAS flat file folder. Other content types XMLs (e.g. articles, preprints, etc) remain unchanged.
Clinical Trials Document-Specific XML tags:
- PUI number for clinical trial documents will be the original NCTxxxxxxxx number for that clinical trial (e.g. <itemid idtype="PUI">NCT00007904</itemid>). This also affects the files name in archives (i.e. LNCT00007904_2.xml in daily update folder)
- The database collection will be “Clinical Trial” (i.e. <dbcollection>CLINICAL TRIAL</dbcollection>).
- The citation type will be “ct” (i.e. <citation-type code="ct"/>).
- The source type will be “c” (i.e. <source type="c").
- The source title and abbreviated source title will be “clinical trials.gov” (e.g <sourcetitle>clinicaltrials.gov</sourcetitle>
<sourcetitle-abbrev>clinicaltrials.gov</sourcetitle-abbrev>).
- The publisher name will be “clinicaltrials.gov” (i.e. <publishername>clinicaltrials.gov</publishername>).
- The <author-group> and <correspondence> elements will be absent for clinical trials.
Fixed Bugs
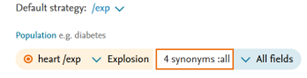
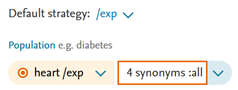
- Fixed an issue in PICO page where repetitive information was added in the term box.
- Fixed highlighting issue where it wasn’t displayed when index term contained a non-alphanumeric character.
- Fixed a bug where when trying to save a search with a reference search between quotes, it will create a corrupted saved search (ie. ‘#2’)
For additional information about Clinical Trials, please refer to the Clinical Trials FAQs.
We are excited to announce that we will be incorporating preprints from the Social Science Research Network (SSRN). This addition will enable us to offer a wider array of cutting-edge research findings that are critical to the evolving landscape of health and life sciences.
Focus Areas
In alignment with the scope of Embase, we will initially focus on two key subject areas from SSRN:
- Health Sciences: This area will cover a diverse range of topics pertinent to public health, healthcare delivery, and clinical practices.
- Life Sciences: This will encompass a variety of biological and neuroscience studies that are essential for advancing research in these fields.
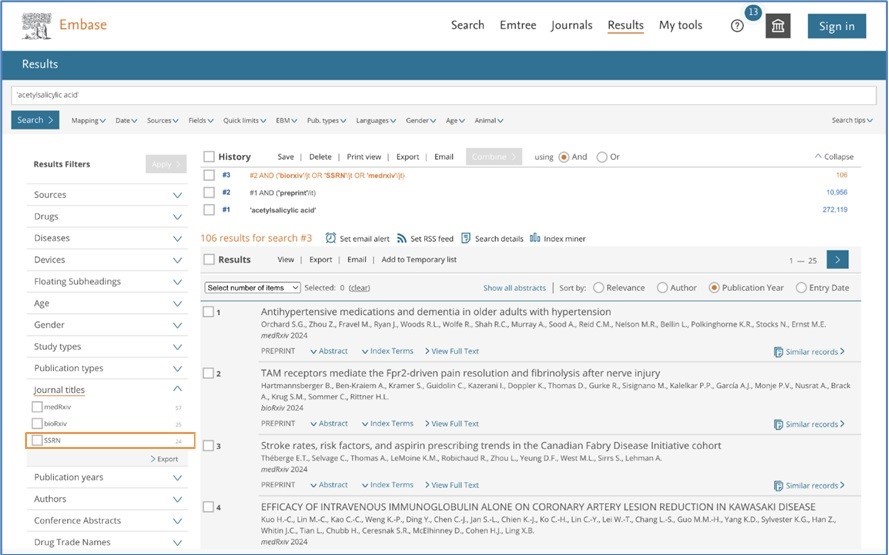
Additional filter for SSRN in Journal Titles
The new SSRN repository can be filtered by limiting the selection to ‘SSRN’ Journal title:
Embase search engine update
From Tuesday October 15, 2024, Embase updated its search engine to the newest version of Elasticsearch (ES). With this move, performance, scalability and stability will be enhanced. It will also allow in the future to explore new exciting capabilities like data visualization with Embase.
We have extensively tested the new search engine update, but we recommend that you test your saved searches and email alerts to ensure they are functioning as expected. If you encounter any issues with your queries, please do not hesitate to contact us.
Update to the PubMed to Embase translation tool
We are excited to announce a new version of the PubMed to Embase translation tool. We have listened to your feedback and make the following changes:
Ability to add/remove terms in the translation box
Once the translation has been performed you will be able to add or remove terms inside of the translation box.
Ability to change the field codes of the translation
When translating non-indexed terms, you’ll be able to change the translated field codes. Just click on the term and select the field code of your choice from the side panel. Note: No field code multichoice is possible for the time being. You can do that by taking the query to the advanced search and modify it there.
Addition of proximity
It is now possible to translate PubMed proximity in the tool. For example, “hip pain”[title/abstract:~1] will be translated to ('hip' NEAR/2 'pain'):ti,ab,kw
After the translation you can click on the translated term and add/remove field codes.
Please note that the terms inside the translation cannot be changed and if you want to change them you need to change them in the PubMed translation box and click on the “Translate” button.
Candidate terms workflow
We have implemented a candidate term workflow. When a MeSH term is mapped to an Emtree candidate term it’ll be properly highlighted. You’ll see a message explaining what a candidate term is and there will be a workflow to change the candidate term to any other Emtree term of your choice if needed.
Other new features
- Translation of & and | to AND and OR respectively.
- Better and more informative error messages.
- PubMed field code “affiliation” is now translated to :ad,ff (Searching in affiliation and address).
Bug fixing
- Terms with AND and OR inside the term are now properly translated.
“sprain and stains”[title] was translated to 'sprain' AND 'stains':ti. Now is translated to ‘sprain and strains’:ti
Feedback and Support:
We value your feedback and are committed to continuously improving your experience with Embase. If you encounter any issues, have suggestions, or need assistance with the PubMed translation feature, please reach out to our support team or use the feedback button at the bottom of the page.
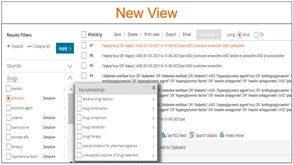
Refreshed look of Clipboards: Now called lists
Clipboard is now called Temporary list and it’s been designed focusing on user experience. Temporary list can hold up to 5000 records and will be deleted once the session is terminated.
- New design has the same features of the old design with a new refreshed look in accordance with the rest of Embase pages.
- Pagination and number of records per page selection have been added to help users who have a large number of records. By splitting the results into multiple pages, it will ensure faster rendering and smoother navigation through the list records. This will save time and enhance the overall experience of using lists.
Sending records to Temporary list
Old view
New view
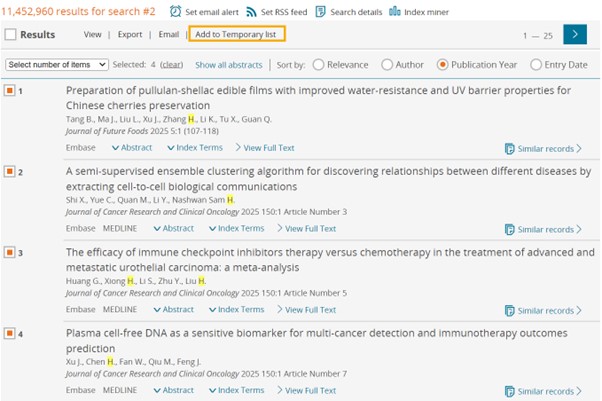
Temporary list vs. Clipboard
Old view
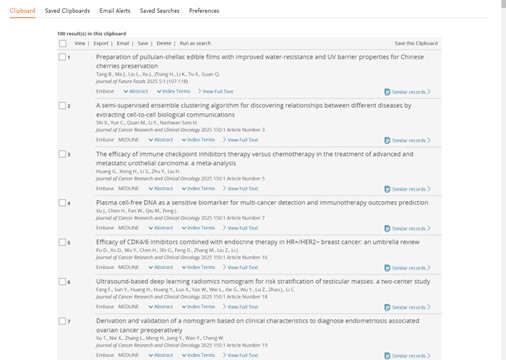
New view
Temporary list can hold up to 5000 records and if you send records that are already present on the list, they will be deduplicated automatically.
From the temporary list page you can Export, Save them a list, Send, Remove or Run the records as a search that will contain the PUIs of the included records separated by OR.

Pagination Allows easy navigation through large number of results set. Faster loading of the results, enables quick access to specific sections within a larger number of results. |
|
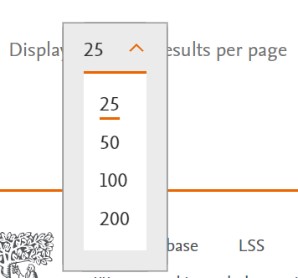
Adjusting the results per page To adjust the number of searches displayed per page, simply click on the dropdown labelled "Results per page." You can choose to display 25, 50, 100, or 200 results per page. For instance, if you choose 25, the first page will show the 25 most recently results. |
|
Saved lists
Keep your records to a Saved list
If you want to save your records in a Saved List (that won’t expire when your session is terminated) you can click on the Save button. Doing that will give you the option to either create a new Saved List or added to an existing one.
Saving records from the temporary list
Old view
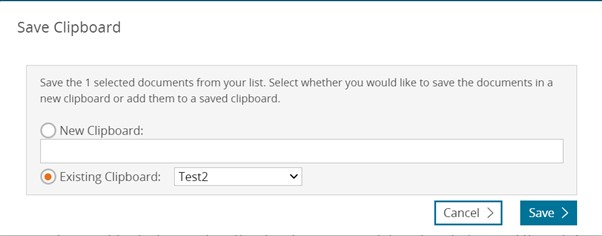
New view
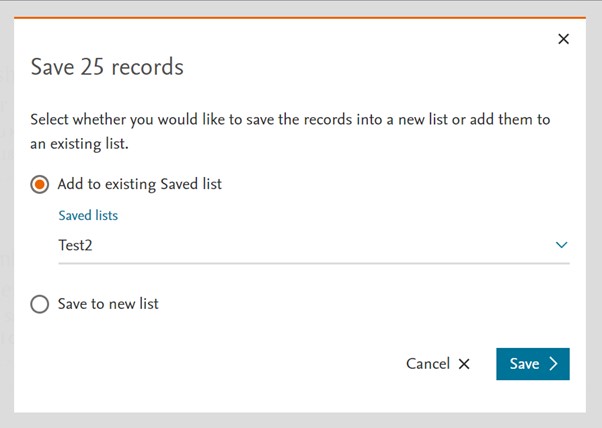
The Saved List page (formerly known as Saved Clipboards) have also been redesigned for a better readability and user experience. Same functionality has been kept.
Saved Lists
Old view
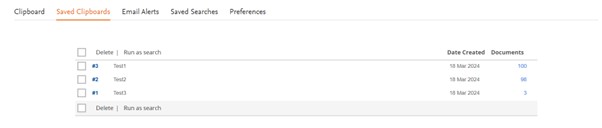
New view
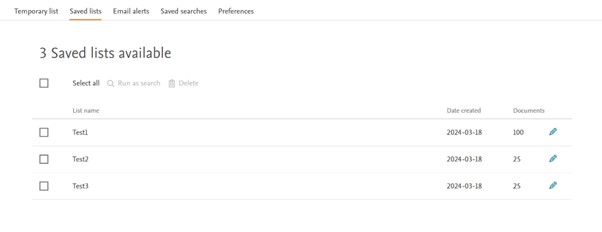
What is the PubMed translator tool?
The PubMed to Embase translation tool is a specialized tool designed to facilitate the conversion of PubMed queries into Embase queries. It offers automated mapping of MeSH terms to Emtree terms, ensuring greater precision when searching in the Embase database. This tool streamlines the process, saving time and reducing potential errors in query translation, making it a valuable resource for researchers and medical professionals looking to access the extensive and focused content available in Embase.
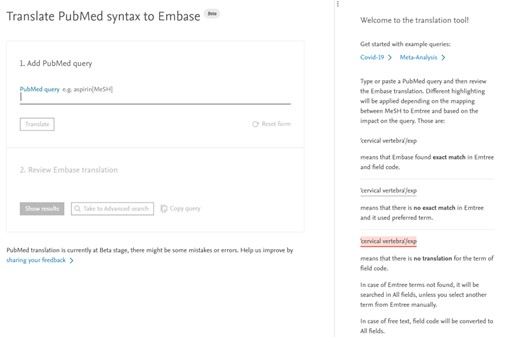
Key Features:
Seamless Transition: It offers a seamless and intuitive platform to effortlessly translate your PubMed queries into Embase queries. No need for manually reformatting your search strings; our tool does it for you.
MeSH to Emtree Mapping: Take advantage of our unique mapping feature that automatically converts MeSH terms to Emtree terms. This ensures that your search is as precise as possible, capturing a more extensive range of relevant literature in the Embase database.
Time-Saving: Save valuable time with our tool, which streamlines the search process. Quickly and accurately adapt your PubMed queries to the Embase database, allowing you to focus on what truly matters - your research.
Unparalleled Database Access: Gain access to Embase's vast database, renowned for its in-depth coverage of pharmacology, drug research, toxicology, and biomedical literature, among other areas.
User-Friendly Interface: Our tool features a user-friendly interface, designed with researchers in mind. Whether you're an experienced professional or a newcomer to Embase, you'll find the translation tool very easy to use.
Limitations of the beta version
The current limitations of the beta version are:
- Once the translation is performed you cannot add or remove terms on the page. In case you want to add/remove terms you can copy the query and modify it in the Embase advanced search or results page.
- Free text field codes cannot be changed. If you want to change the translated field codes, you’ll need to copy the query and modify it in the Embase advanced search or results page.
- Not all the field codes are accepted. Please refer to the tables of accepted codes below.
- Proximity search is not yet implemented. Searching for "hip pain"[Title/Abstract:~2] will be translated into "hip pain"[Title/Abstract].
- Dates should be entered with “/” symbol (YYYY/MM/DD). Support for “-” will be added in another version.
- Pharmacological action is not yet implemented.
- Boolean operators inside a phrase search are going to break the phrase (ie. 'salt and pepper'[ti] will be converted to 'salt' AND 'pepper':ti
Support How to use it?
Click here to find out how the tool works and what the translation mappings are.
Feedback and Support
We value your feedback and are committed to continuously improving your experience with the PubMed translation tool. If you encounter any issues, have suggestions, or need assistance with the new translation feature, please fill out the survey that pops up when clicking on the 'sharing your feedback' button at the bottom of the page.
Stay tuned for more exciting updates coming soon!
Release 1
Improved search precision with the ‘controlled clinical trial’ EBM limit.
The "Controlled Clinical Trial" EBM limit can be accessed from the Results page, Quick Search, Advanced Search, Drug Search, Disease Search, and Device Search forms.
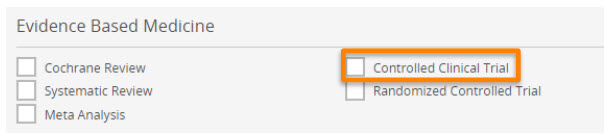
Controlled clinical trial limit pertains to original clinical trial reports incorporating a control group (e.g., placebo, sham, or no treatment, standard intervention) for comparison with the experimental intervention.
With the updated definition of the limit, the search will only pick the records where the controlled clinical trial has been duly recognized and indexed. The retrieved records will be considerably fewer than before, but will have a higher level of precision.
- Old definition
[controlled clinical trial]/lim → 'controlled study'/exp AND 'clinical trial'/exp - New definition
[controlled clinical trial]/lim → 'controlled clinical trial'/de
Editing email alerts
When creating an alert, if a user accidentally includes spaces between miltiple email addresses, our system failed to validate the recipient information.This created an issue when the user wished to edit the saved email alert.
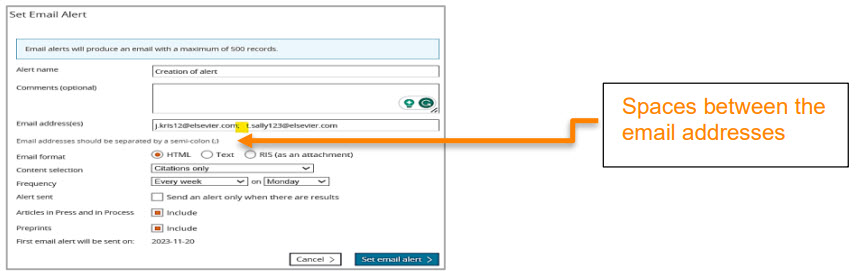
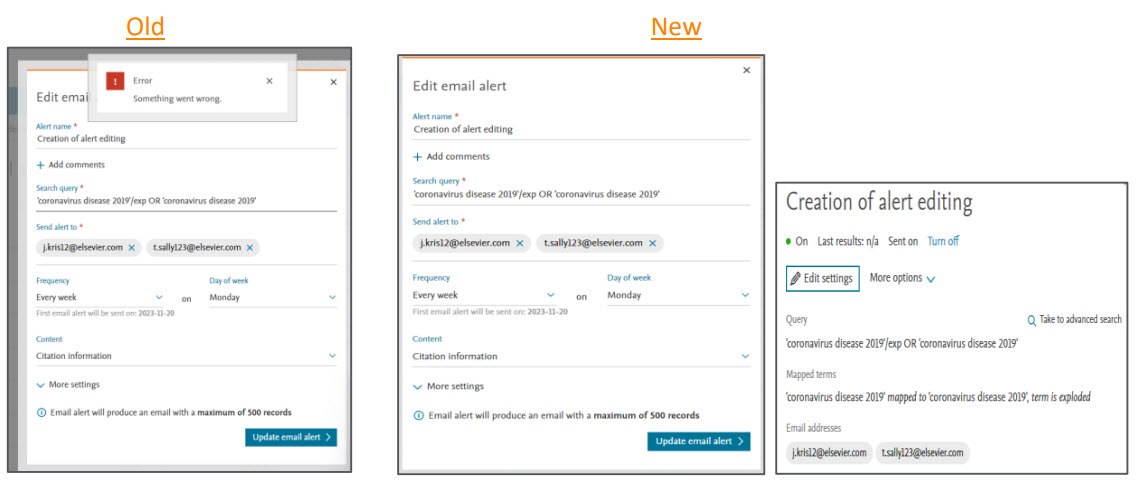
To address this, we have implemented an automatic space-trimming to ensure that the recipient information is saved correctly and it does not cause issues while editing the saved email alert.
Longer Embase session
When using Embase.com, if a user perform a search and leave the site and returns after 30 minutes, user will experience an automatic implicit relogin if they have the 'Stay signed in' option selected. However, users who don’t have this option checked will need to enter their credentials and log in again.

In this release, we have extended the user session from 30 minutes to 2 hours, even for users who haven't chosen the 'Stay signed in' option.
This enhancement ensures that the session remains active up to 2 hours, which means user won’t need to re-login if the idle time is less than 2 hours.
Bugs Fixed
Correction to the unknown database collection in the Embase
Content within Embase that intersects with the nursing collection is marked with the 'NURSING' tag.
Changes will be observed in -
XML exported file
Old → <dbcollection code="NURSNG">unknown</dbcollection>
New → <dbcollection code="NURSNG">NURSING</dbcollection>Results page
In addition to Embase, will find article also marked as ‘NURSING’ for the overlapping articles

Record details page
Source of indexing will also include NURSING for the overlapping articles.

- Highlighting was breaking for index terms containing consecutive spaces, resulting in search failures. To address this, we have implemented a normalization process for these index terms before applying highlighting. As a result, searches will now run without any problems.
Release 2
Why do you change the indexing?
We need our indexing technology to be more flexible and modular for future innovation (new content types, new information extracted from existing content).
What changes exactly?
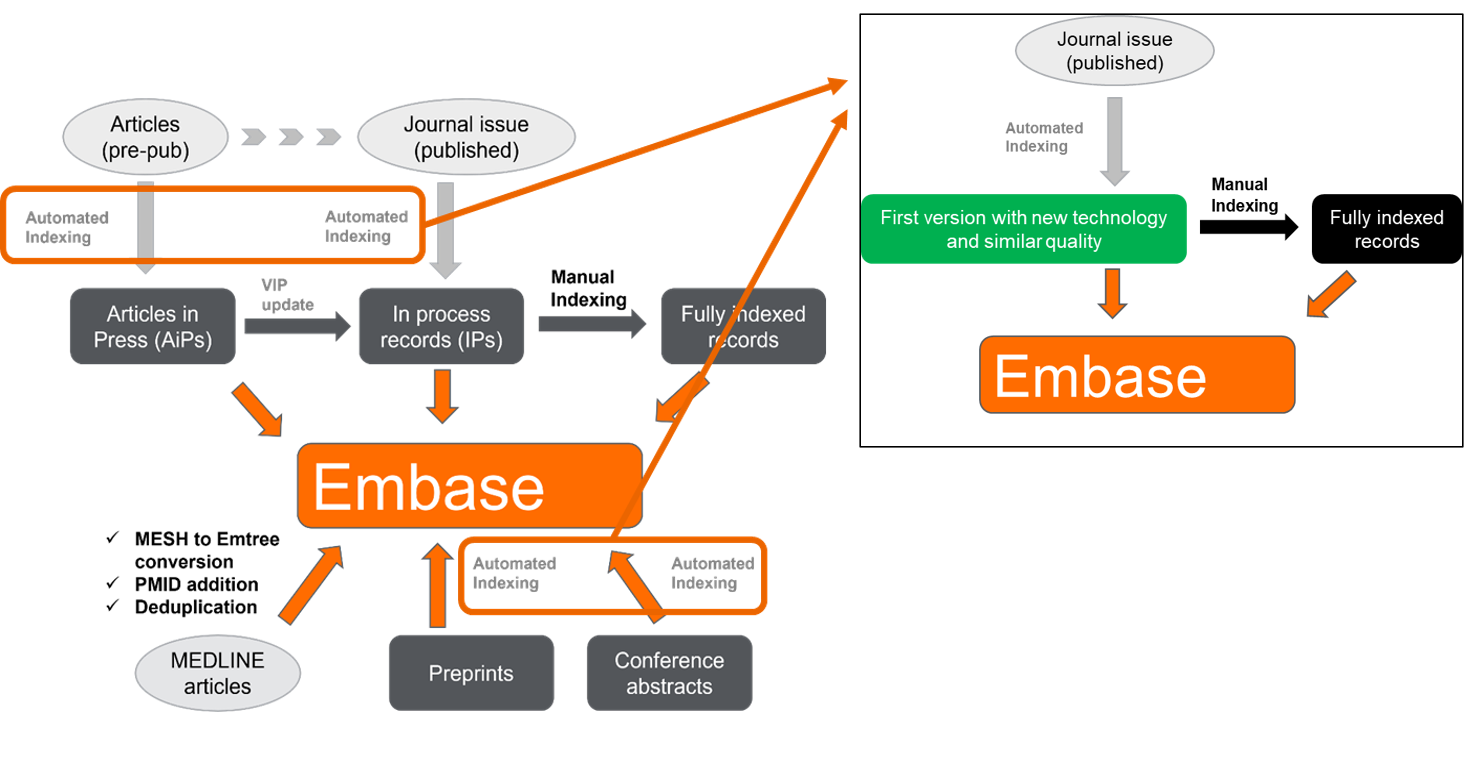
We are improving the technology we use for the automatic indexing step of processing Embase’s records. For this, we are switching to a different technology: SciBite with its named entity recognition (NER) and extraction engine TERMite.
This technological improvement is the first step to providing our customers with improved indexing quality. This enhancement has been released on November 20th.
What stays as it used to?
- No change in format.
- Indexing Embase involves a two-step process for most articles (preconditions are explained in our indexing guideline): the first step is automatic indexing on the title and abstract of the Embase article. Then, after verification the preconditions, the next step is an in-depth manual indexing based on the content in the full text, usually available a few weeks later. This will continue to be done as it always has been, and as documented in this FAQ.
- This new technology will deliver a similar indexing quality (recall and precision) compared to our previous process which also leverages Emtree terms to automatically index content, making the Embase content available as soon as possible to customers. You will not need to make any changes to your workflow. In a comparable way to any time our indexing is updated (e.g., during the 3x/year Emtree releases), we recommend reviewing saved search/email alert queries and update them if needed.
How does this look like concretely?
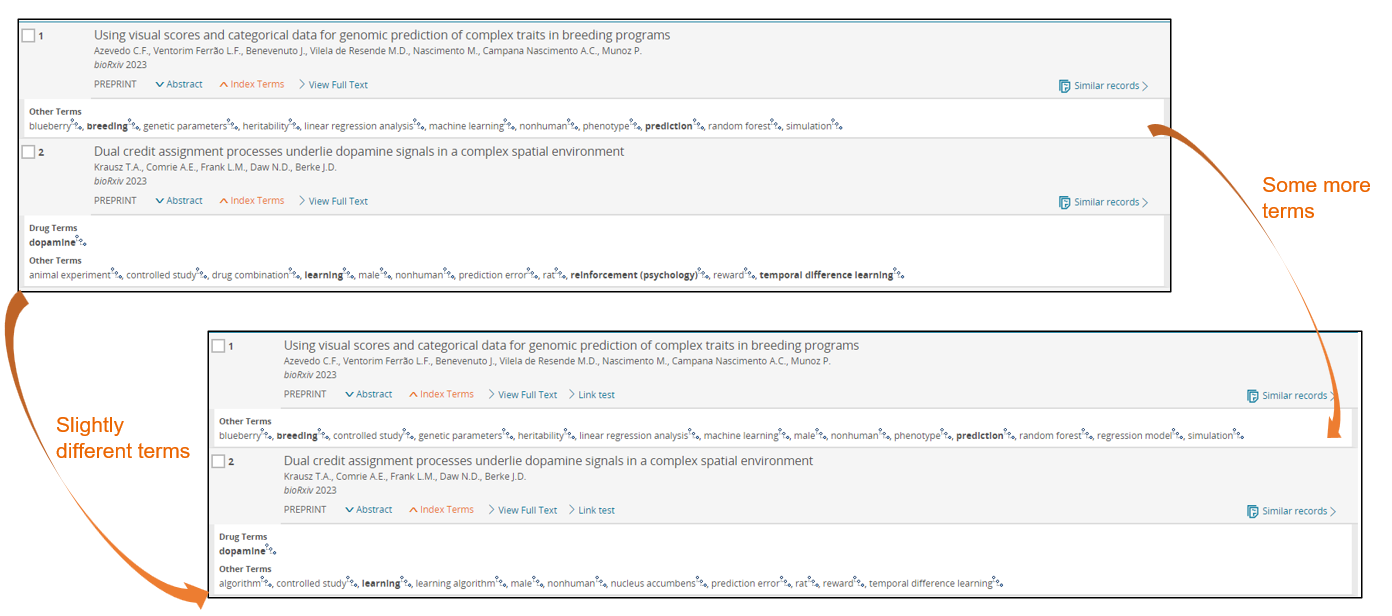
Here are two examples where you can see that there can be slight differences with the new technology indexing more terms, different terms, or less terms.
What will happen in the future?
The next step will be for the initial indexing (on titles and abstracts) to be improved and complemented with medical devices, tradenames, and manufacturers from the full text article. This will enable our customers to retrieve the most relevant records sooner to improve precision and recall at an earlier stage while manual indexing is still performed in a second step. This is an improvement that our medical devices customers have been requesting. On a long term, the new technology will also allow the processing of new content types (new sources or new extracted information), which will provide further advancements in the upcoming years.
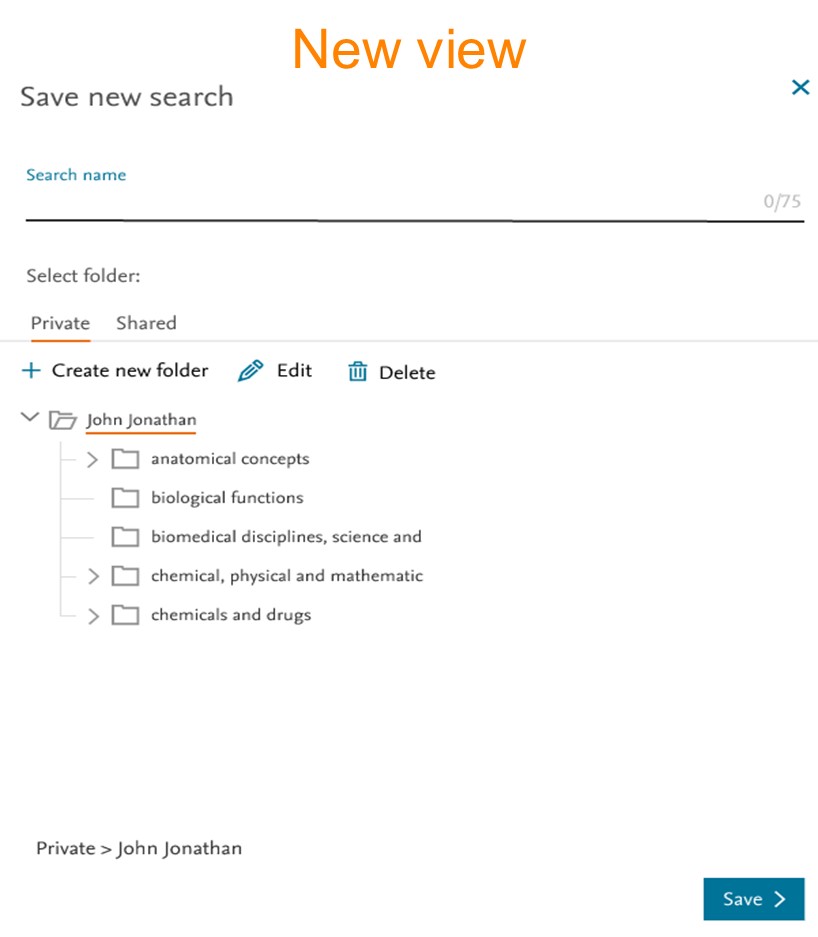
1. Saved search page: Simplified and more Intuitive
New saved search page focuses on improving user experience, folder organization, and manging saved searches in an efficient manner.
- New design introduces filter which allows users to quickly locate specific searches by name. This feature adds convenience, enabling users to find the desired search or search group more easily without scrolling through a long list.
- The new design will make it easier to identify the relationship between the saved search and the folder. Highlighting the folder names provides a visual cue that aids users in understanding where their searches will be saved. This prevents any confusion or accidental misplacement of searches.
- Pagination has been added to help users who have a large number of saved searches. By splitting the results into multiple pages, it will ensure faster rendering and smoother navigation through the list of saved searches. This will save time and enhance the overall experience of using saved search page.
Creation of saved search
Naming the search is optional and can only be done when saving the search. Names are limited to 75 characters. |
|
By default, the Private Folder and the Shared Folder only contain one folder, with the user’s name. The following options are available to add, rename or delete folders.
- To create a folder, simply click on the '+ Create new folder' option, make sure that you have selected the desired parent folder where you want the new folder to be placed.
- To edit/modify folder name and description users can select the ‘Edit’ option.
- Selected folder can be deleted using the ‘Delete’ option.
Managing saved searches
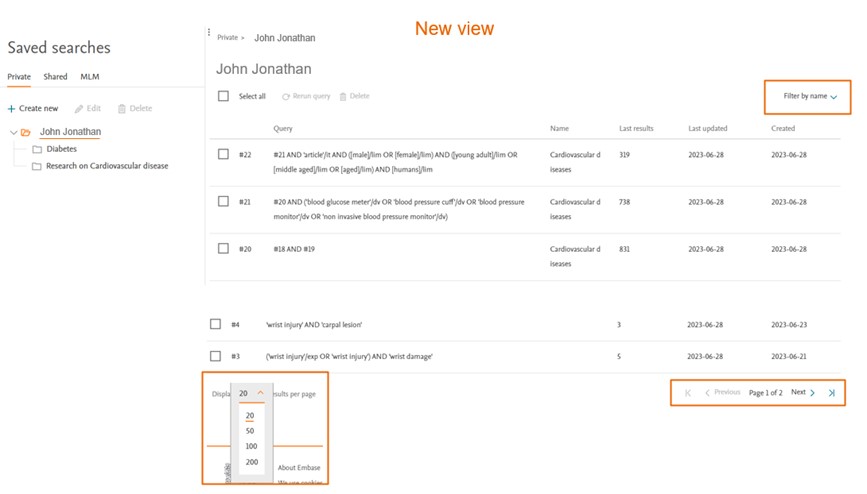
The newest saved searches will be at the top, and older saved searches are at the bottom of the list.
Some new features introduced are -
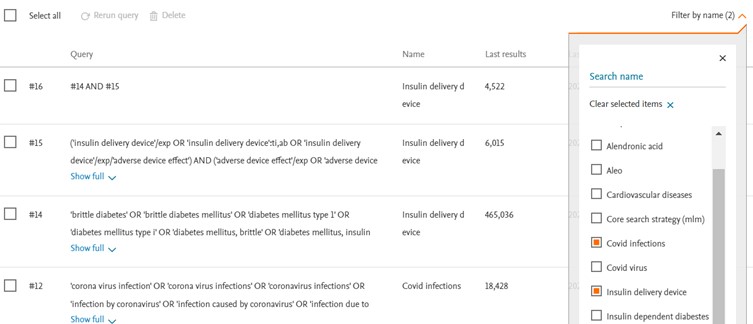
Filter by name
The filtering by name feature is advantageous for users who maintain their saved searches within a single folder. When users assign specific names to their saved searches, the filter by name functionality enables them to locate the desired saved search or group of saved searches more quickly and efficiently. This saves time by reducing effort in finding the exact search they need within their collection of saved searches.
Users also have the ability to choose multiple search groups, the results displayed will only show the selected searches.
Pagination
Allows easy navigation through large number of results set. Faster loading of the results, enables quick access to specific sections within a larger number of saved searches. |
|
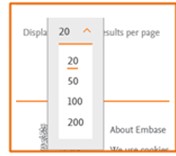
Adjusting the results per page
To adjust the number of searches displayed per page, simply click on the dropdown labeled "Results per page." You can choose to display 20, 50, 200, or 500 searches per page. For instance, if you choose 20, the first page will show the 20 most recently saved searches. |
|
Alphabetic Sorting of Folders and Subfolders
The folder and sub folders will be sorted in alphabetical order.
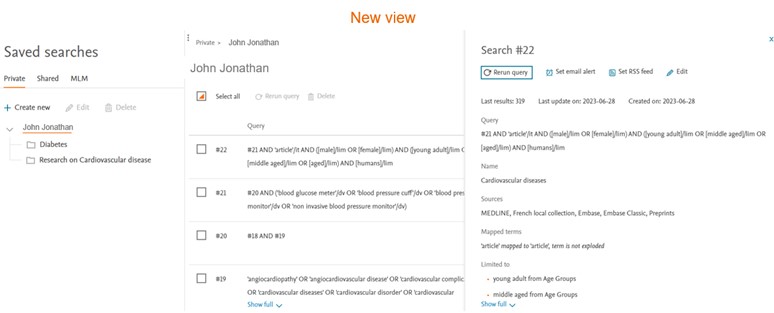
Search details panel
To review more details of the saved search, please click on the search of interest. The search details panel will appear on the right side of the screen. All the actionable buttons like ‘Set email alert’, ‘Set RSS feed’ and ‘Edit’ are moved to the details panel.
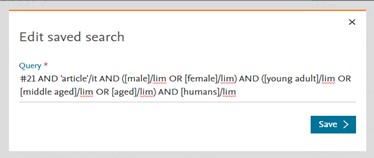
Edit search window
This dedicated edit window allows users to review and modify their search easily. |
|
2. Consistency between information displayed in email alert and record details page
The Email Alert and Record Details page provide essential information regarding the 'Country of Author' and 'Entry Date.' Here are the details regarding each:
- Country of Author: Refers to the correspondence address of the author
- Entry Date: The entry date indicates two significant milestones in the record's life cycle within Embase. Firstly, it reflects when the record was first introduced in Embase, which may occur when the article is in the "Article in Press" or "In Process" stage. Secondly, it denotes when the record was fully indexed, known as the "Full Record" stage.
Post this change, for the articles with multiple correspondence addresses, all of the corresponding countries will be included in both the record details page and email alert.
For e.g L2022640464
Old | New | ||
Email alert | Country of author, in case multiple correspondence address is available | United States, Germany | United States, Germany |
Entry date | 2023-02-17 (Full record)2023-03-08 (Full record), 2023-02-17 (Article in Press/In process) | 2023-03-08 (Full record), 2023-02-17 (Article in Press/In process) | |
Record details page | Country of author | United States | United States, Germany |
3. Improving stability of Embase API
Embase API is not recommended for large volumes of data downloads. Large volumes of data download cause instability and performance issue. Hence in order to give all users an equal opportunity to use search on Embase API, we have established limits to 500k on how much data can be downloaded.
Bugs Fixed
- Issue with Clipboard export fixed. When records are added from multiple searches into the clipboard, on export incomplete result are downloaded. This issue has been fixed.
- Editing Search Line in Results page always showed blank (-) while using the test query feature. This issue has been fixed

1. Laying the foundation for COUNTER compliance
Embase is aiming to be COUNTER compliant in 2023. As the first step towards achieving this goal we have defined and implemented tracking for the COUNTER metrics. Once the reports are audited, we will host the customer facing reports on EPIC system. Please watch this space for more information on timelines on availability of Embase COUNTER compliant reports.
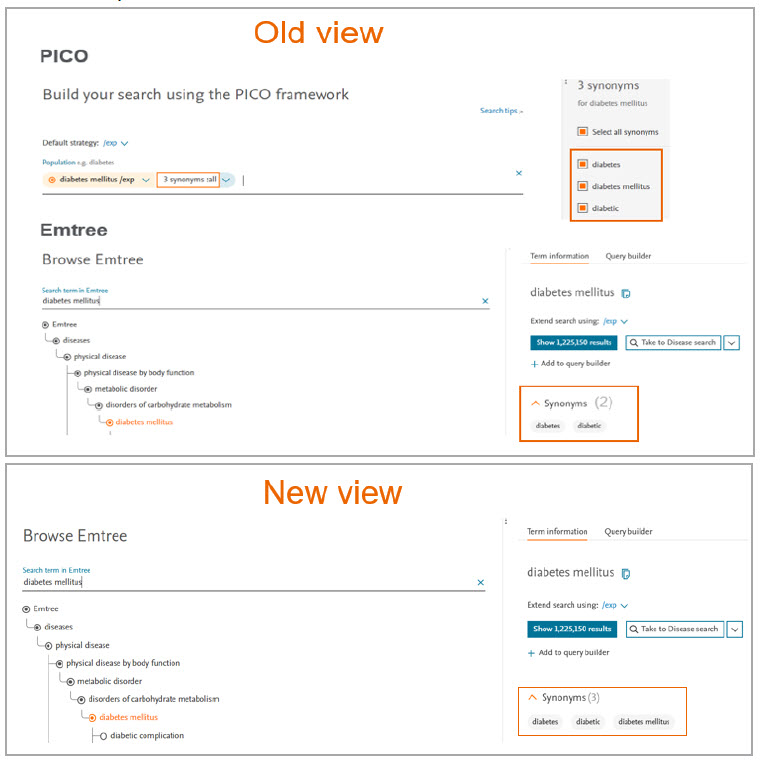
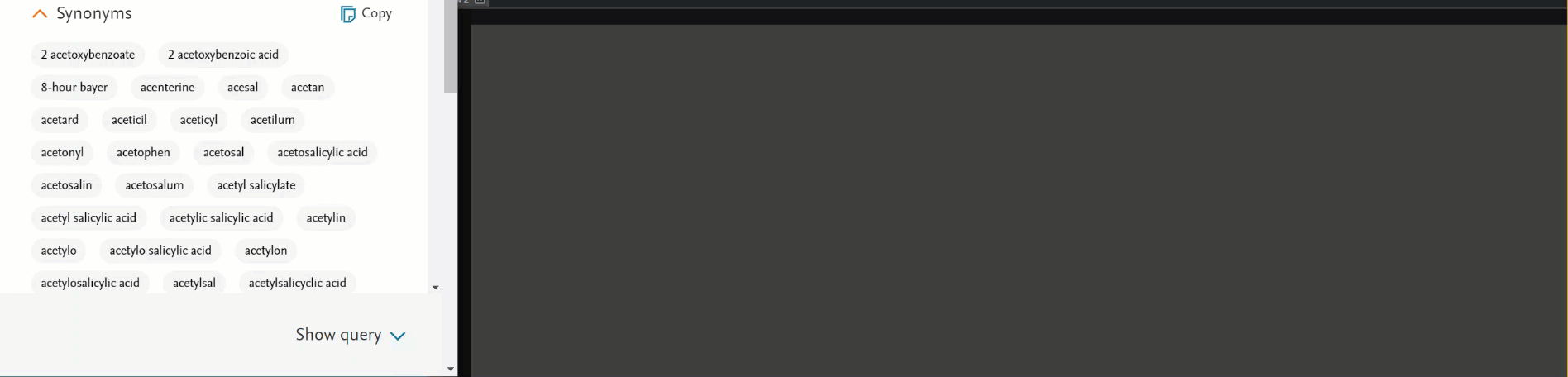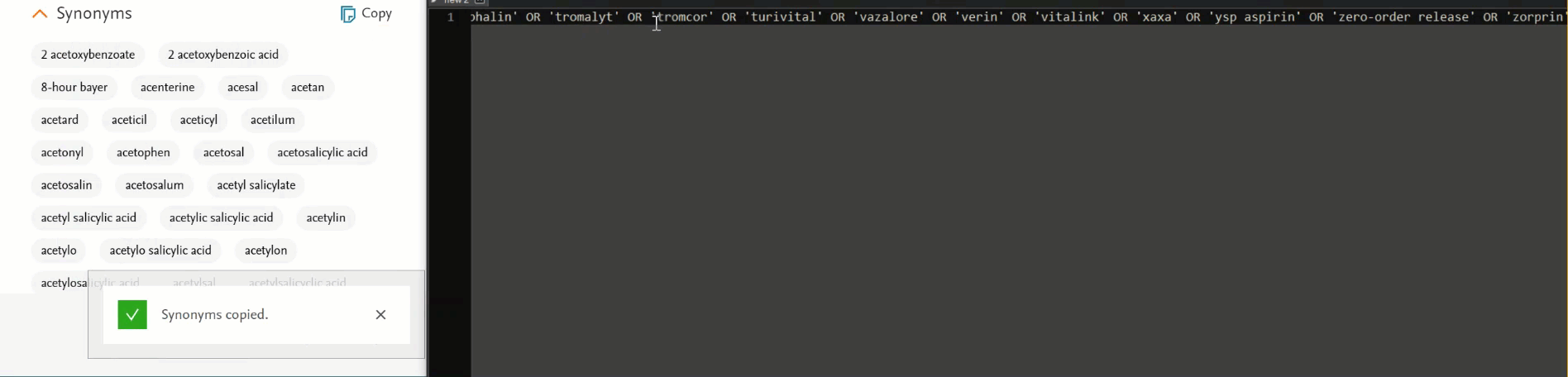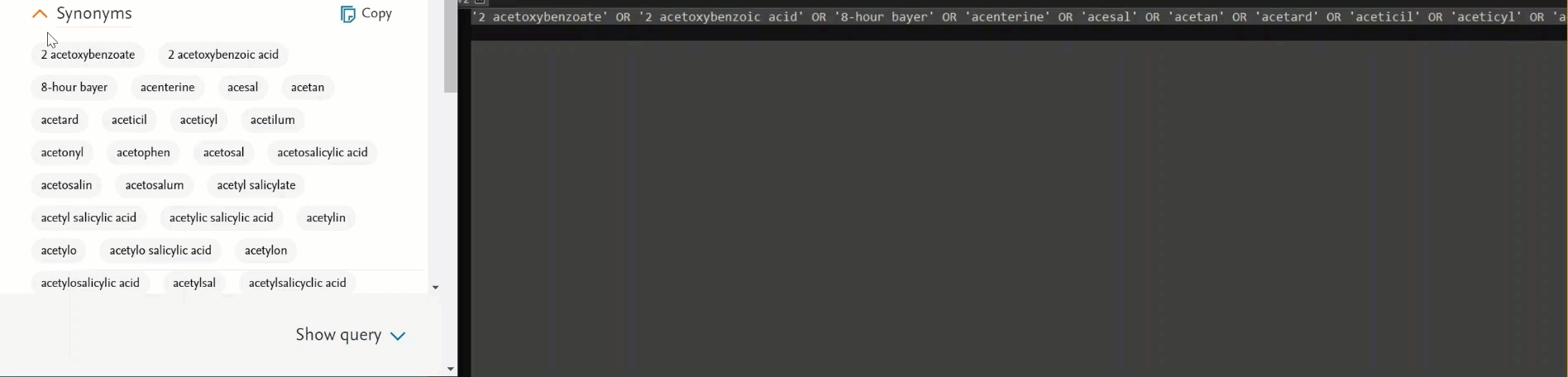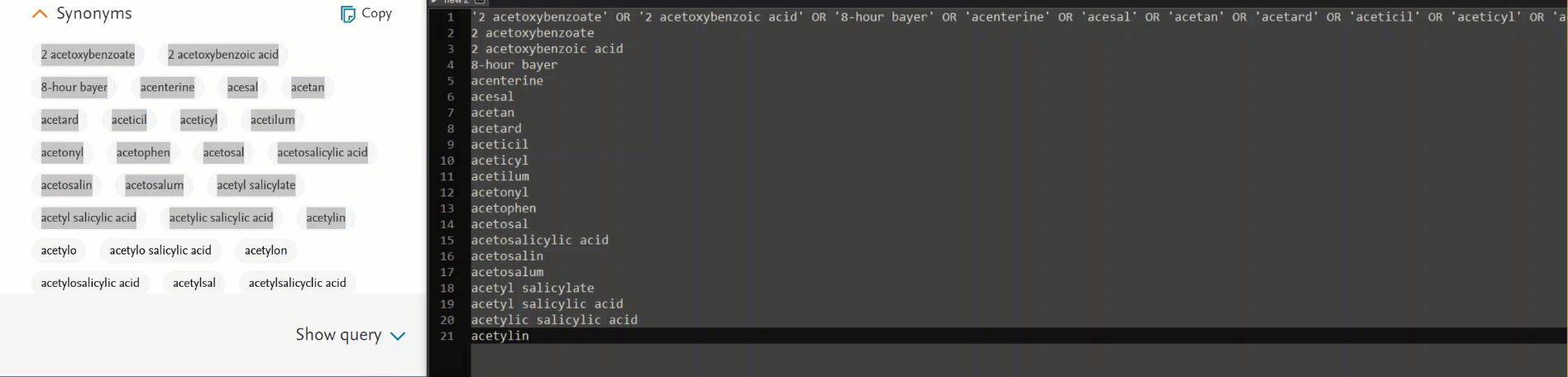
2. Synonym list on Emtree page updated
Previously, the search created using the synonym list in PICO vs search created using the synonym list in Emtree resulted in mismatch number of results. It was because the searched term was not part of the synonym list in Emtree.
In this release we have added the searched term in the synonym list of Emtree for result consistency.
Bugs Fixed
- Fixed Embase session interruptions caused due to third party plugins like RSS reader.
- Missing GMDN information added in Emtree for the term ‘Sjoegren's antibody test kit’.
- User were unable to save an alert with search query of size 8k characters This issue has been fixed.
Easy to use Emtree page
Split view display allows easier navigation
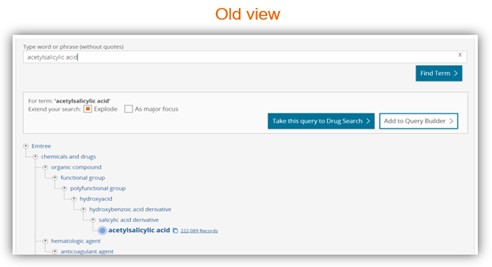
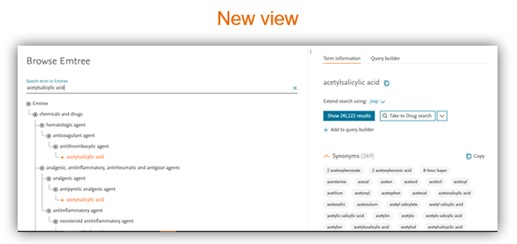
- New Emtree page design with a split view and with a new tree behaviour which allows an easier navigation.
- Addition of the option to add a /syn modifier that maps, explodes, and searches your term adding all the available synonyms as free text in all fields.
- Ability to copy synonyms either with an OR operator between the terms to use them as part of your search or as a list for your reporting purposes.
- Split view to see all the time the Emtree on the left panel with an easy navigation system and information regarding synonyms, Dorlands Dictionary, GMDN information and CAS registry number.
- CAS registry numbers now have a link to Reaxys and PubChem.
- When you select the term, Embase will automatically detect the type of term it is and suggest where to send the search (Disease, Drug, Device or Advanced). Now user can change that suggestion if they wish so.
You can now extend your search using different modifications to search the term directly, send it to Drug, Disease, Device, Advanced search, or to the Query Builder:
- /mj (Focuses your search to records where your search term is indexed as "major focus")
- /de (Searches your term or maps to the preferred Emtree term (if your term is a synonym in Embase). No narrower terms are added.
- /exp/mj (Searches your term (or maps to the preferred Emtree term) and related narrower or narrower terms but where the terms are indexed as "major focus")
- /exp (Searches your term (or maps to the preferred Emtree term) and related narrower or children terms)
- /br (Maps, explodes and searches your term as free text in all fields)
- /syn (Maps, explodes and searches your term adding all the available synonyms as free text in all fields)
Copy the synonyms of terms in two different ways:
- Click on “Copy” button to copy all the synonyms with the OR operator between them. Useful when you want to extend your search to all the synonyms.
- Select all the synonyms by click and dragging and do a copy/paste action will paste the synonyms as a list.
Unified the behaviour of the pages that have Emtree in them
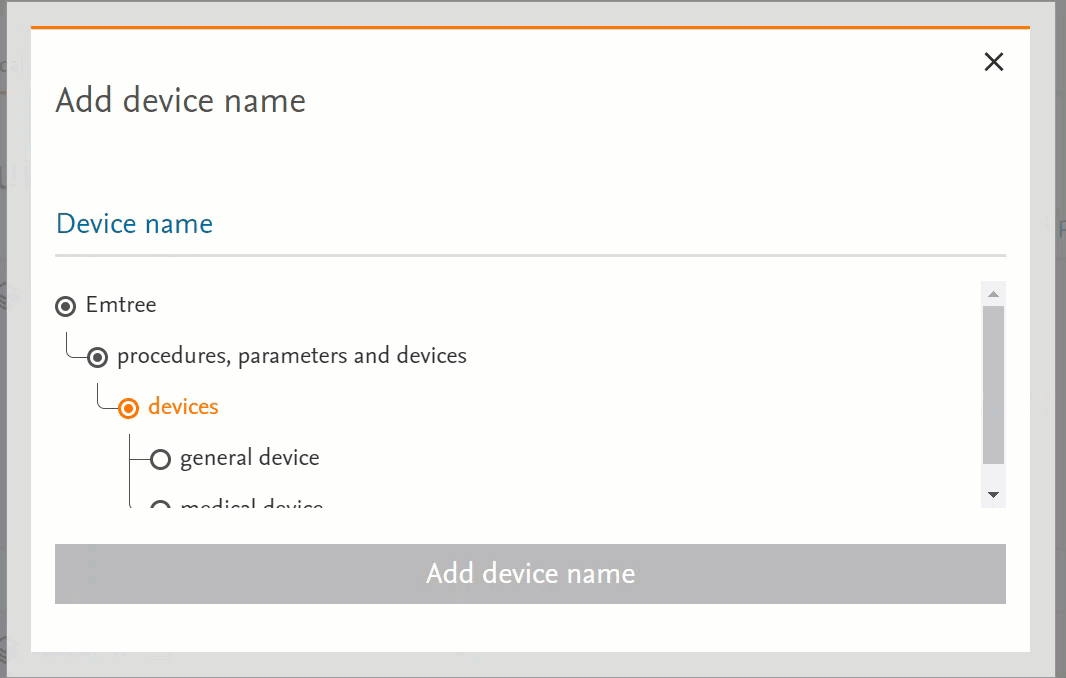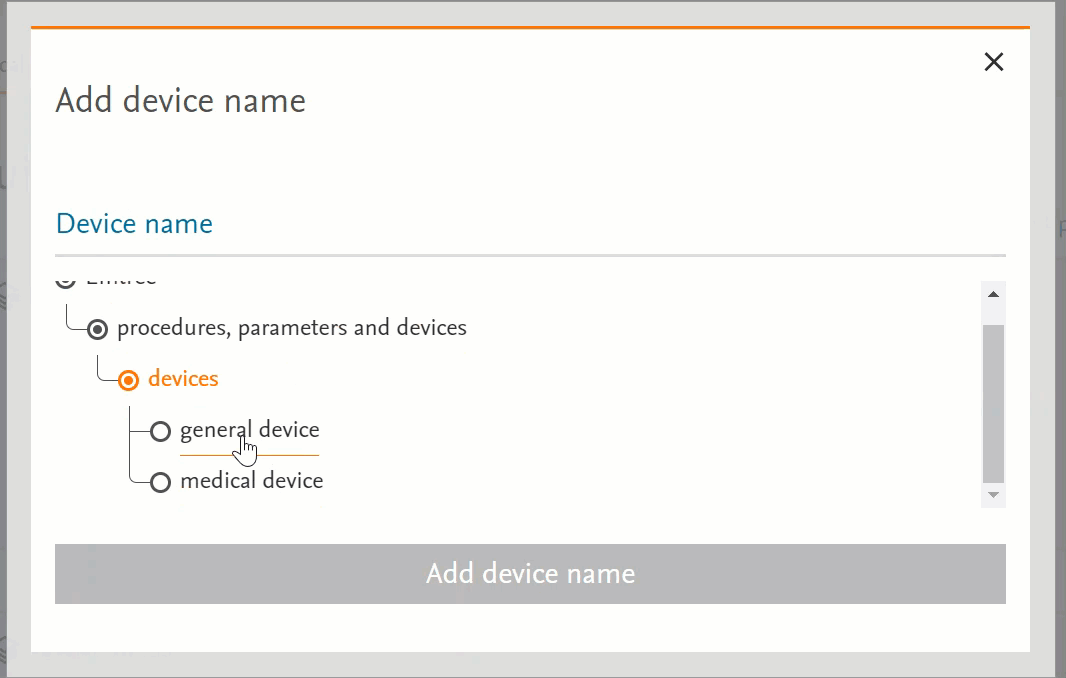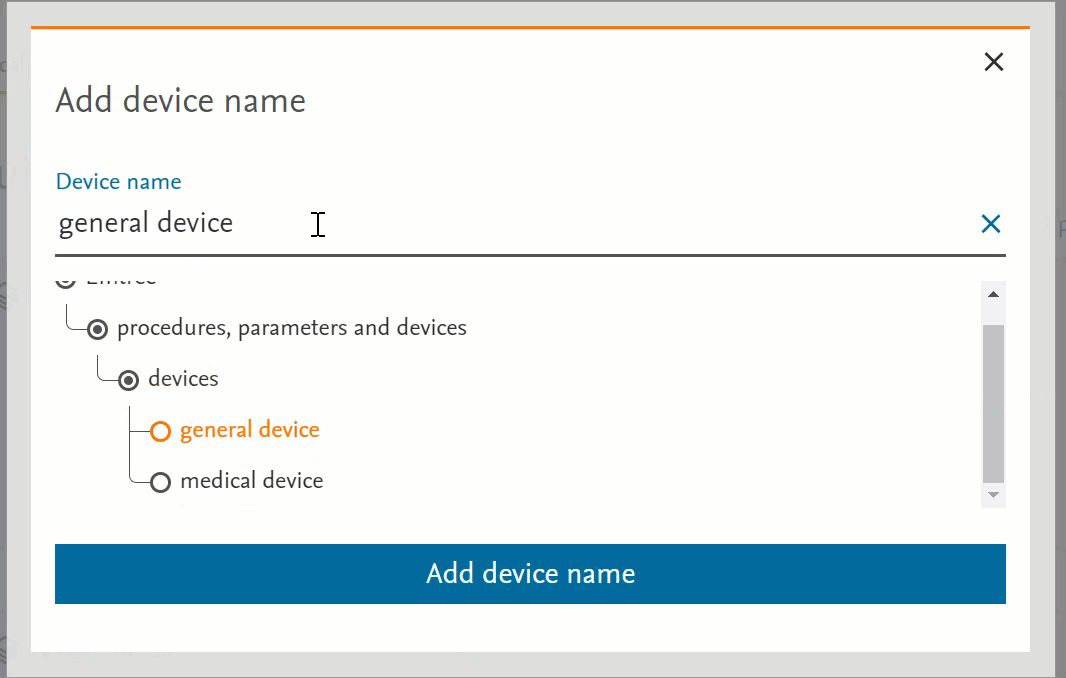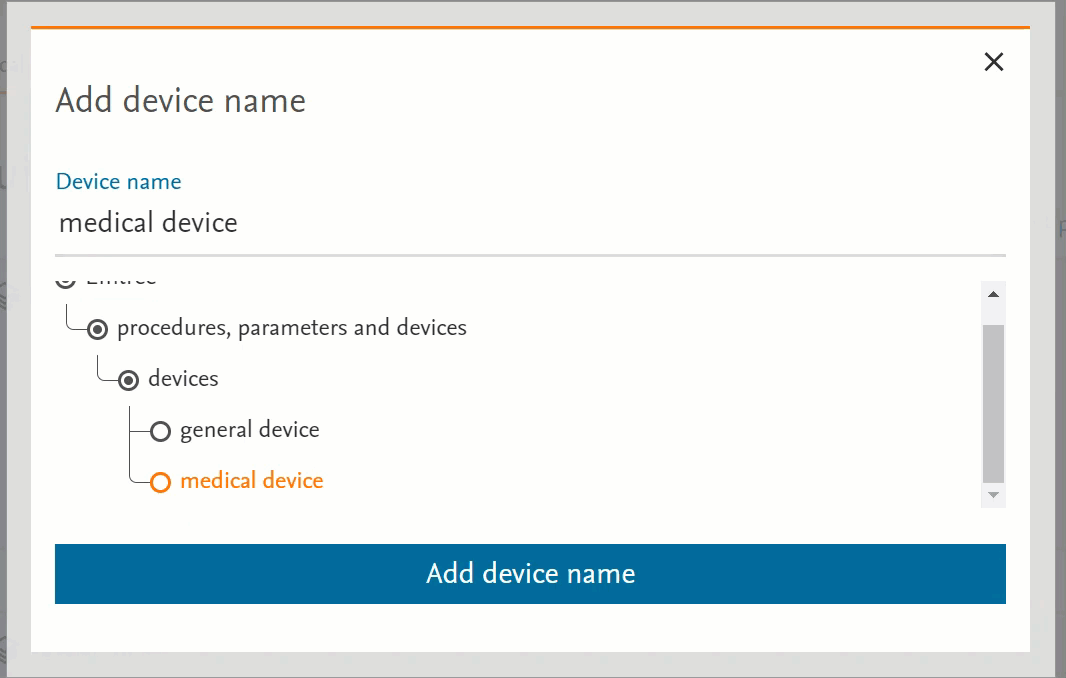
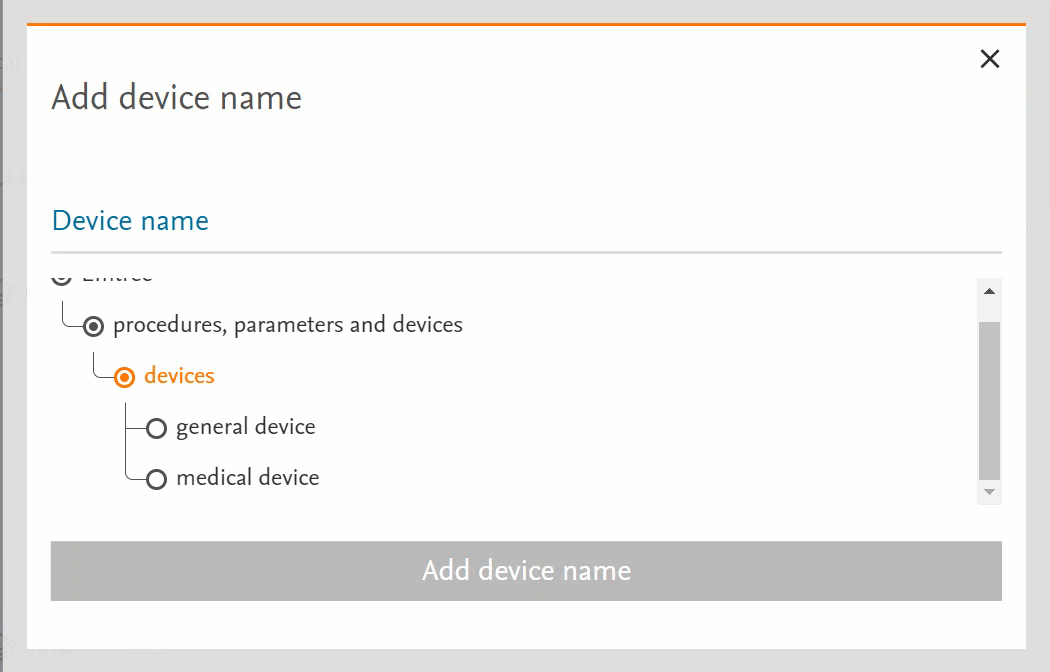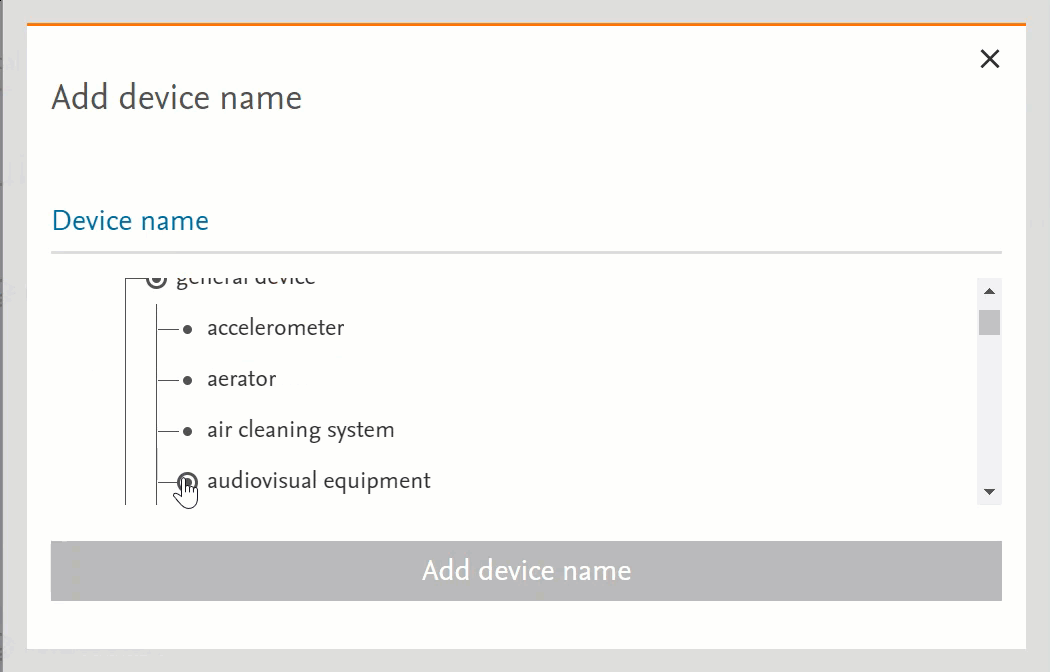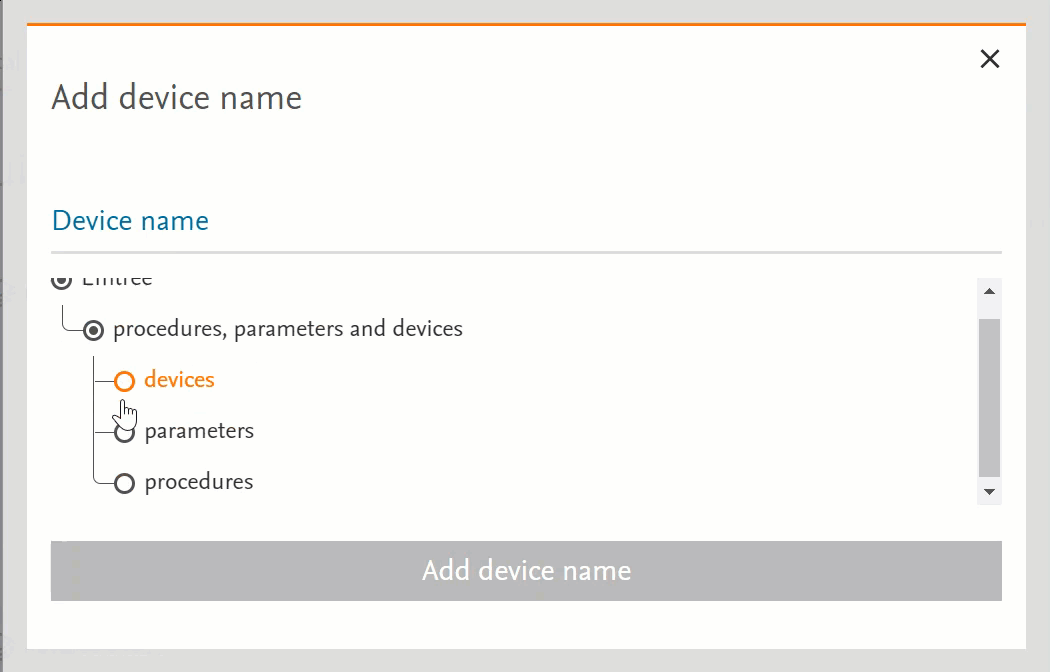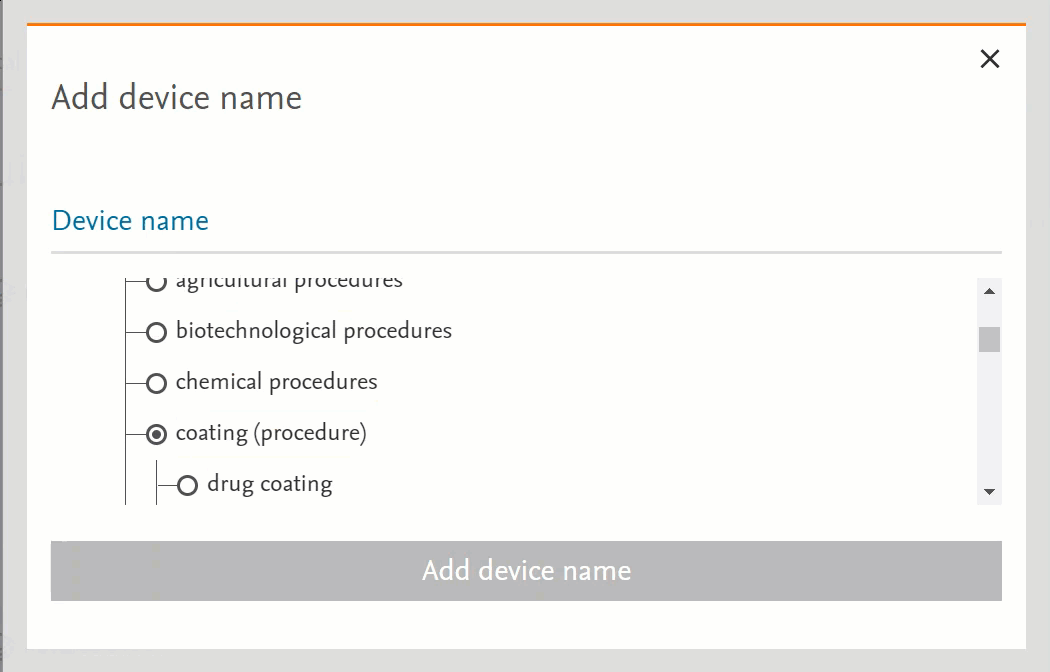
- Clicking on the term will send the word into the search bar (PICO or Medical Device pages) or in the right-side panel with more information (Emtree page):
- Clicking on the circle at the left of the term will open the terms under the selected term:
- If there’s no circle next to the term but just a black dot means that that term does not have any narrower terms.
Linking of preprint to related published article
Post the inclusion of preprint records in Embase, we received feedback from the users to provide linking between preprints and its related published article. We have added indication to the results page, and the email alert of the existence of the related publication.
Results page
Preprints with related published article will display a new line below source with text: Published article DOI: xy
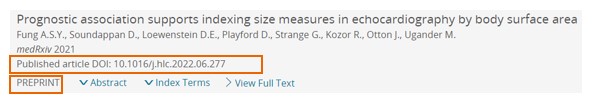
Record details page
Prepints with published article will include an info banner on top of the preprints with text: This preprint is now published, DOI: xy

DOI information of the corresponding published article is displayed in the ‘Associated DOI’ field in the additional section of the record details page.
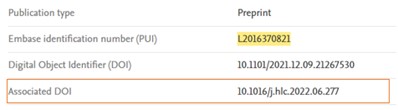
Email alerts
For the alerts which include preprints, a new line will be displayed for the preprints with related published article below full text URL with highlighted text: This preprint is now published, DOI: x

Valeriana MLM search strategy updated
We have updated the MLM drug Valeriana. This is available in the Saved search section in ‘My tools’.
Author highlights available in MS word/pdf file
When the user searches for author and if a match is found, the matched author name will be highlighted in the exported MS wors/PDF document.
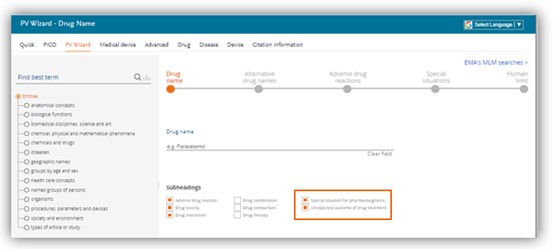
Default subheading in the PV Wizard
The subheadings ‘Special situation for pharmacovigilance’ and ‘Unexpected outcome of drug treatment’ subheadings are checked by default in the PV wizard search form.
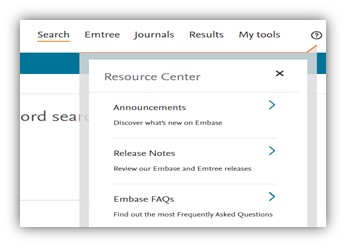
Embase in-product help center
We are introducing an in-product help center in Embase. This will be available by clicking on the ‘?’ symbol on the top right-hand corner of the product. You can easily access all product announcements, onboarding, and FAQ content within the product. The new product release notifications will be pushed through the in-product help center.
New date field introduced in API/DAAS
We have introduced a new date field called ‘record updated date’ (/ud), which captures all the updates made to the Embase record. This field can be used to query and retrieve all the daily updates in the API.
Bugs Fixed
- PUI will be available in the RIS export file even when the search only contains PUIs
- Editing a saved search will not result in changing the names of all saved searches
- The search containing field code ‘df’ and ‘dn’ when combined with the other searches resulted in errors. This issue has been fixed.
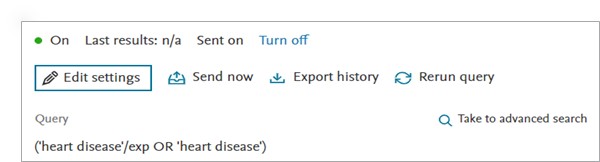
User friendly ‘Alert history’ & ‘Journal’ page
We have designed a user friendly alert history page which provides user an overview of the available alerts in their ‘Email alerts’ section.
Following information is available to the users in the Email alerts page -
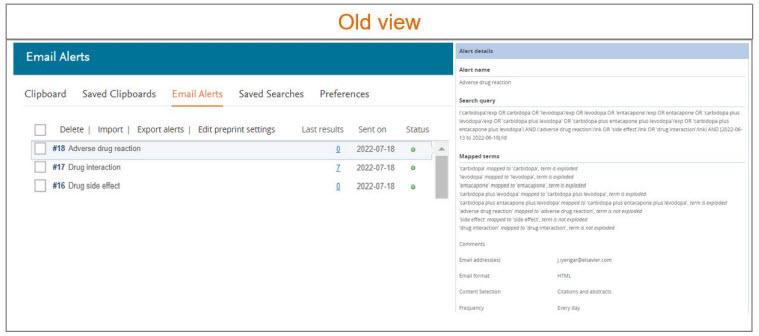
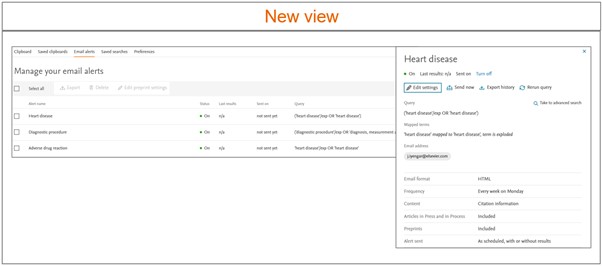
To review more details of the alert, please click on the alert of interest. The alert details panel will appear on the right side of the screen. All the actionable buttons to modify the alert are moved to the ‘Alert details’ panel.
Take to advanced search – Will copy your query to the Advanced Search page where you can edit and rerun your search
Edit Alert Window
We have updated the edit alert window to showcase the most important settings at the top and the advance settings can be reviewed by clicking on ‘More settings’ option.
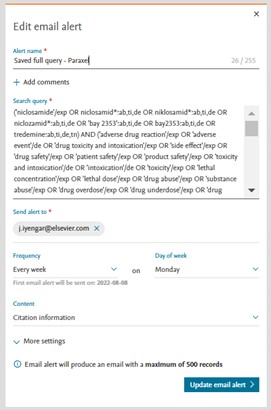
More advanced settings
Field name | Function |
|---|---|
Comments (optional) | This will be the name featured in the email alerts list and the subject of the email alerts. |
Email address(es) | Enter the email address for alert notifications.
You can also enter email addresses of colleagues. |
Frequency |
|
Content selection |
|
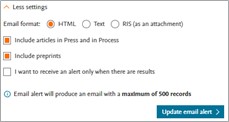
Email format | Select the format of your alert email:
|
Include articles in Press and in Process | Tick this box to also include Articles in press and in process to your search results. |
Include preprints | Tick this box to also include preprints to your search results. |
I want to receive alerts only when there are results | Tick this box if you’d only like to receive alerts when there are results for your search. |
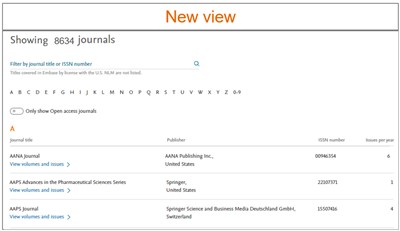
You can view our new and improved Journal page, by clicking on the 'Journals' in the top bar of any page. These journals are sorted alphabetically. It includes all the indexed journals in Embase. This journal list will be updated 3 times a year.
Note: There may be Journals which were supported in the past and have now been deactivated, since we still have indexed content from the past it will be available in the Journal list.
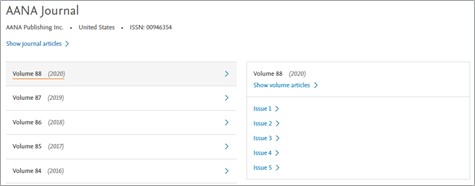
New functionalities -
Volumes and issue page
All the volumes are displayed in the descending order, with the most recent ones at the top and the old ones at the bottom. Clicking on volume will show the list of the issues.
Additional functionalities -
In order to standardize searching by date we have extended support for multiple date format searching.
Following table provides information of the additional date format supported for searching per date field
Format supported | Loaded date (:ld) | Conference date (:dc) | Publication date (:pd) |
|---|---|---|---|
dd-mm-yyyy (e.g) | '20-10-2020':ld | '20-10-2020':dc | '20-10-2020':pd |
yyyy-mm-dd (e.g) | '2020-10-20':ld | '2020-10-20':dc | '2020-10-20':pd |
mm-yyyy (e.g) New | '10-2020':ld | '10-2020':dc | '10-2020':pd |
yyyy-mm (e.g) New | '2020-10':ld | '2020-10':dc | '2020-10':pd |
YYYY (e.g) New | '2020-10':ld | '2020-10':dc | '2020-10':pd |
Range - [dd-mm-yyyy TO dd-mm-yyyy] (e.g) New | [01-01-2020 to 31-12-2020]/ld | [01-01-2020 to 31-12-2020]/dc | [01-01-2020 to 31-12-2020]/pd |
Range - [yyyy-mm-dd TO yyyy-mm-dd] (e.g) New | [2020-01-01 to 2020-12-31]/ld | [2020-01-01 to 2020-12-31]/dc | [2020-01-01 to 2020-12-31]/pd |
Bugs fixed
- Managing alerts made easy in the Alert history page
- Alert name – Name of the created alert
- Status of the alert – ‘On’ for the active alerts and ‘Off’ for the deactive alerts
- Last results - number of results sent in the last email alerts. If results sent > 0, the user on clicking on the last results will be directed to the results page running a search which produced the last results sent.
- Sent on – Date when the last alert was sent
- Query – The search query of the created alert
- Turn off – Clicking on this will deactivate the selected alert
- Turn on – Clicking on this will activate the selected alert
- Send now – Results since the alert was last run until now will be sent to all email addresses specified in the settings of the selected alert. Using this feature does not affect the sending of the scheduled alerts.
- Export history – Retrieve details of all the changes made to the created alert. The exported csv contains ‘Alert name’, ‘Event’ ( help track modification to the alert), Date and time (GMT) – when the event occurred, ‘Search query’, ‘Records included’, ‘Frequency’, ‘Receipient’ and ‘Status’
- Rerun query – The query wil run in Embase and you will be taken to the Results screen
- Edit settings – Will open the ‘Edit email alert’ window and allow you to edit all options you gave in when creating the alert
- Easily find all the Journal covered by Embase
- Search box - To find a journal of interest you can use the search box at the top of the page by entering the Journal title or the ISSN. To filter the results you can also pre select the starting alphabet or the digit of the Journal title below the search box.
- Only show Open access journals - displays Open access journals
- View volumes and issues – For more information on the journalclick on this option
- Show journal articles – User will be directed to the Results page, the search will retrieve all records associated to the selected journal. For eg – For the journal AANA Journal, clicking on the link will execute the following search '00946354':is OR 'aana journal'/jt
- Show volume articles – User will be directed to the Results page, the search will retrieve all records associated to the selected journal and volume. For eg – For the journal AANA Journal, clicking on the link will execute the following search ('00946354':is OR 'aana journal'/jt) AND '88':vi AND '2020':py
- Issue # - User will be directed to the Results page, the search will retrieve all records associated to the selected journal, volume and issue. For eg – For the journal AANA Journal, clicking on the link will execute the following search ('00946354':is OR 'aana journal'/jt) AND '88':vi AND '2020':py AND '1':ip
- Standardize searching of dates in Embase
- Users able to create an email alert from saved searches
- Highlighting of keywords to be available in the exported file (PDF/Word)
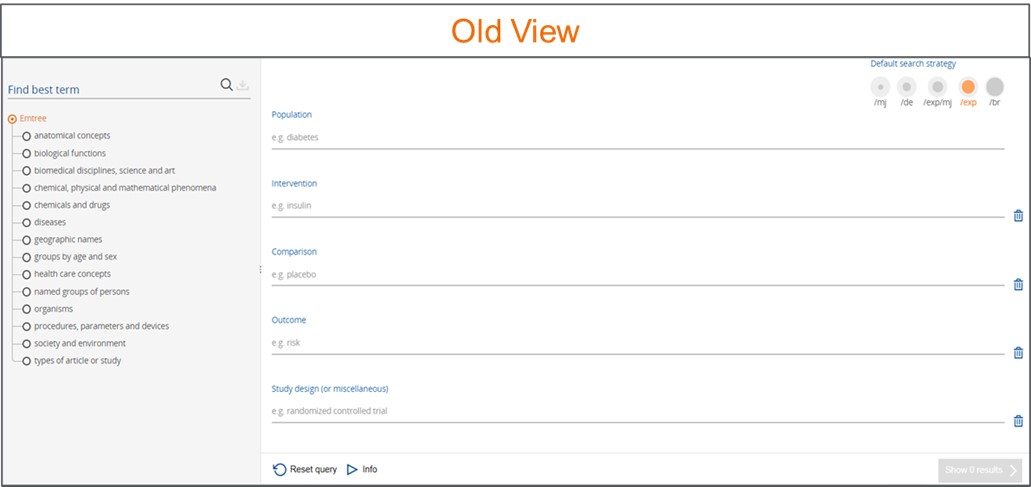
1. Narrowing search results made easy in PICO form
We have brought the PICO search form front and center and moved the Emtree panel to the right side of the form. You now have the option to apply limits directly in the PICO form.
You can build focused questions using 5 elements of the PICO search (Patient, Intervention, Comparison/Control, Outcome, Study type). This search form will combine terms with an OR operator between each search term within the same element and an AND operator to combine terms of different elements.
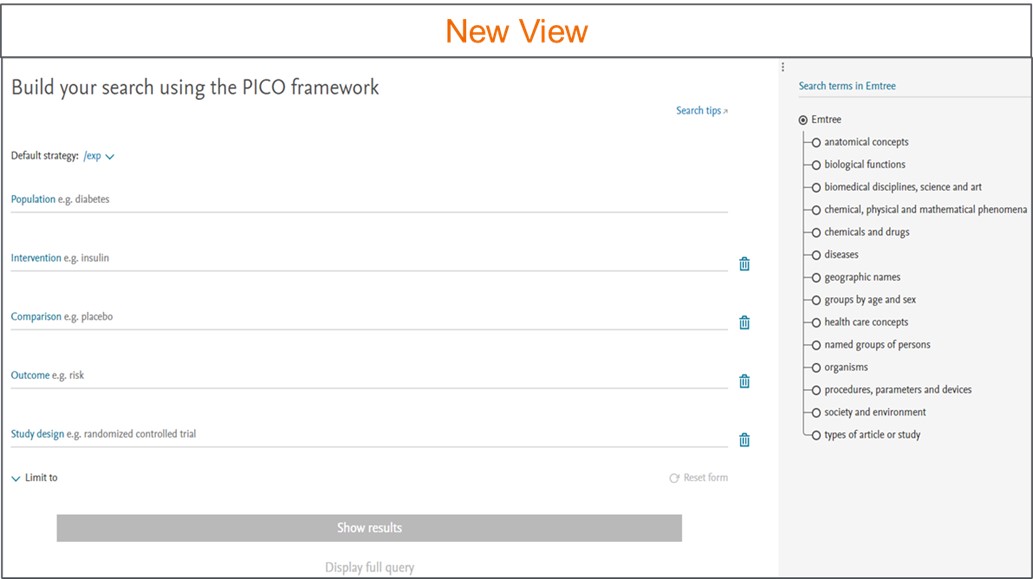
You can directly type into these fields in the PICO search form or find the best term in the Emtree panel on the right. The added term will be highlighted blue if they are not preferred terms in Emtree and orange if they are. Use ‘Add # synonyms’ to see and add or remove synonyms for your search term.
At the top of the screen, you can choose from 4 different search strategy options from the drop down. These options can also be selected from the drop-down arrow next to each search term:
- /mj (Major Focus): Focuses your search to record where your search term is indexed as major focus.
- /de (Index term): Searches your terms or maps to the preferred Emtree term (if your term is a synonym in Emtree).
- /exp (Explosion): Searches your term (or maps to the preferred Emtree term) and related narrower or children terms.
- /br (As broad as possible): Maps, explodes and searches your term as free text in all fields.
You can also clear fields or remove fields or reset the form entirely at any time.
Apply limits directly in the PICO form -
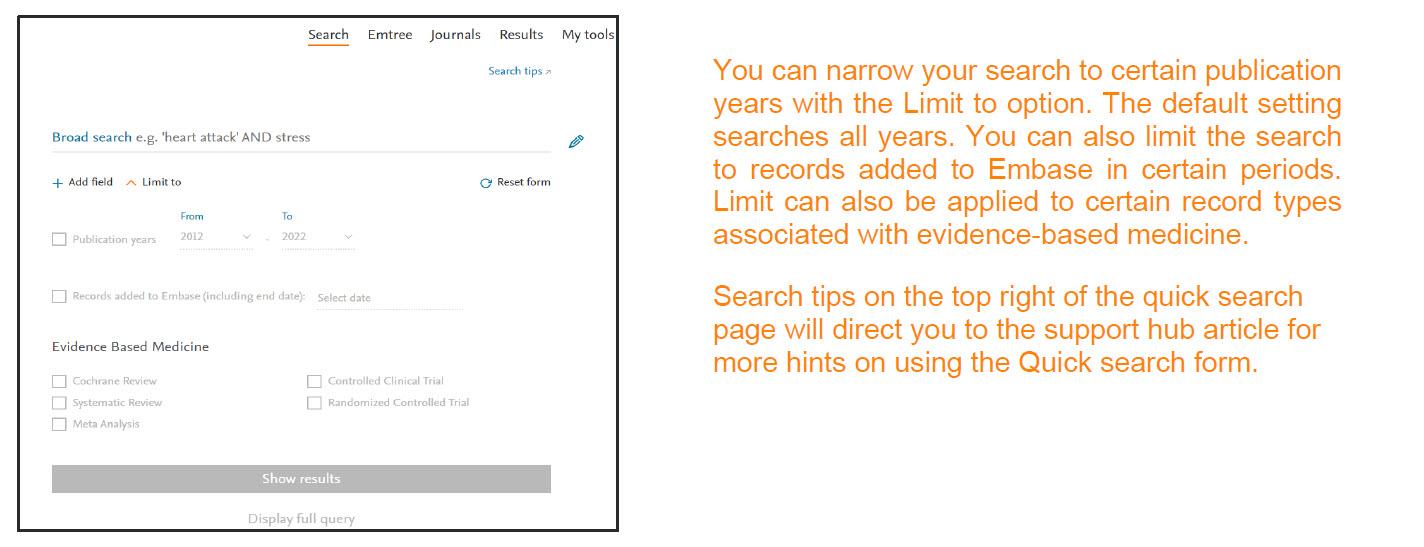
You can narrow your search using the Limit to option. You can search for records within a certain publication year or for records added to Embase in certain period.
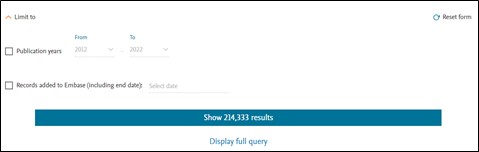
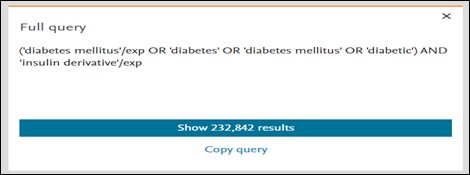
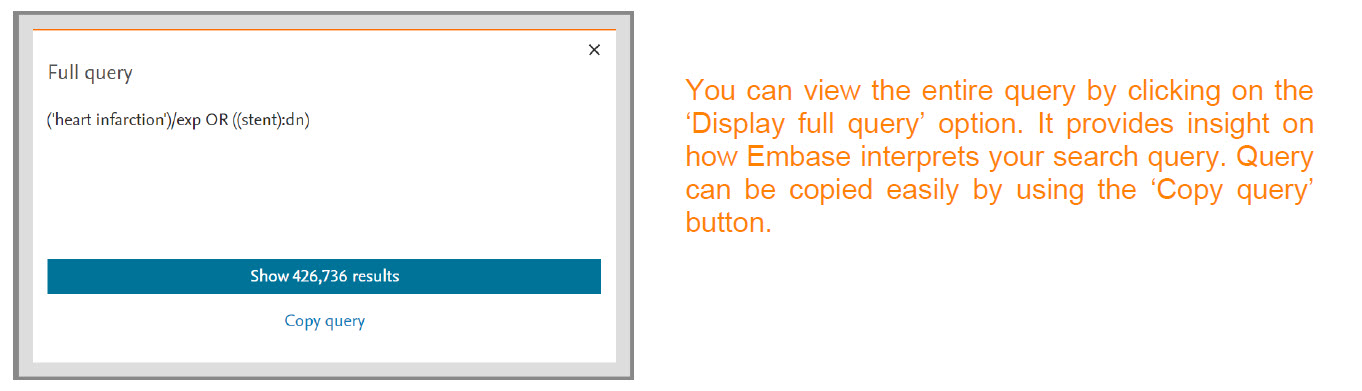
You can view the entire query by clicking on the ‘Display full query’ option. It provides insight on how Embase interprets your search query. Query can be copied easily by using the ‘Copy query’ button.
2. Evidence based medicine limits updated
For better accuracy we have updated the definition of ‘Cochrane review’ and the ‘Controlled Clinical trial’ EBM limits to the following.
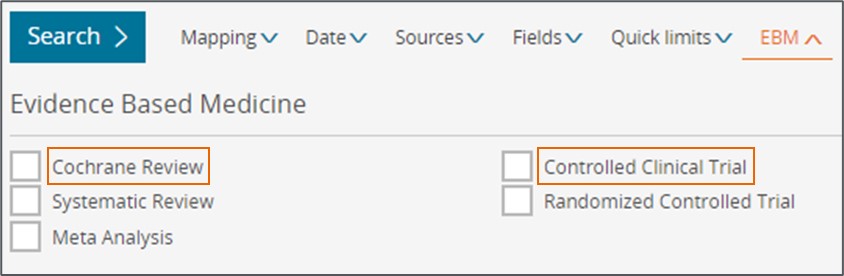
Name | Old mapping | New mapping |
Cochrane review | ‘1469493X’:is | (‘1469493X’:is OR ‘14651858’:is) |
Controlled Clinical Trial | 'controlled study'/exp AND 'clinical trial'/exp | 'controlled clinical trial'/de |
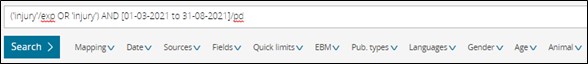
3. Allow searching by date range with the publication date field code (pd)
We will now support searching by date ranges using the Publication date field code.
For e.g In order to retrieve all the literature associated to the term 'injury' which was published between 1st March 2021 and 31st August 2021.
You can carry out the search ('injury'/exp OR 'injury') AND [01-03-2021 to 31-08-2021]/pd.
4. Record details page to display information more clinical trial registries
CTN and registry links from additional clinical trial registries will be available in Embase record details page.
Additional registries covered in Embase are –
Registry | Country |
ANZCTR | Australia/ New Zealand |
ReBEC | Brazil |
CRiS | South Korea |
CTRI | India |
RPCEC | Cuba |
IRCT | Iran |
JapicCTI | Japan |
jRCT | Japan |
UMIN-CTR | Japan |
JMACCT CTR | Japan |
PACTR | (South) Africa |
SLCTR | Sri Lanka |
TCTR | Thailand |
NTR | Netherlands |
Bugs fixed
|
|
- The overlapping of the search number in the history on Drug, Disease and Device key subheading window is fixed.
- Articles in which searched term is preceded with a single quote in abstract was not retrieved in the past. This bug has now been fixed.
- Hits will be highlighted in the record details page when the search is performed with field modifiers like Emtree subheading (lnk), accession number (an), Medline PMID (ui) and PUI (id)
- Full Record field will now only include the Full Record entry date and not the AiP/In process date in the export file.
- Logged in users can now export more thank 20k records.
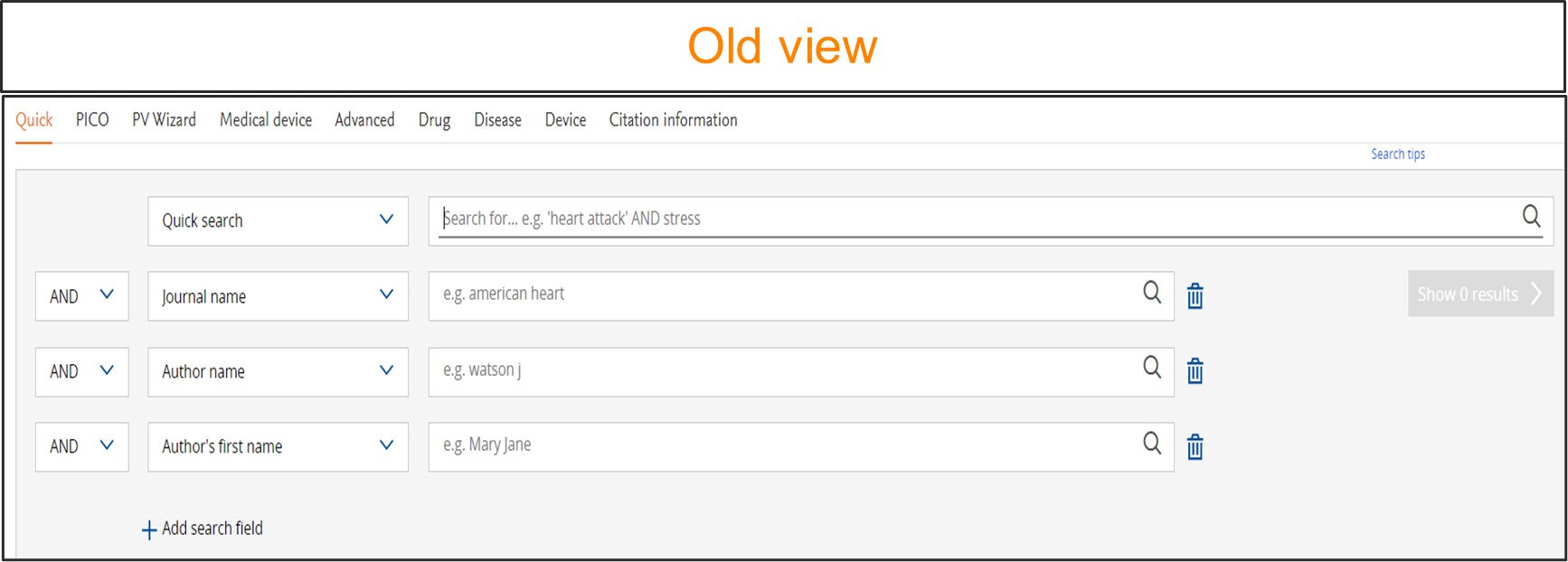
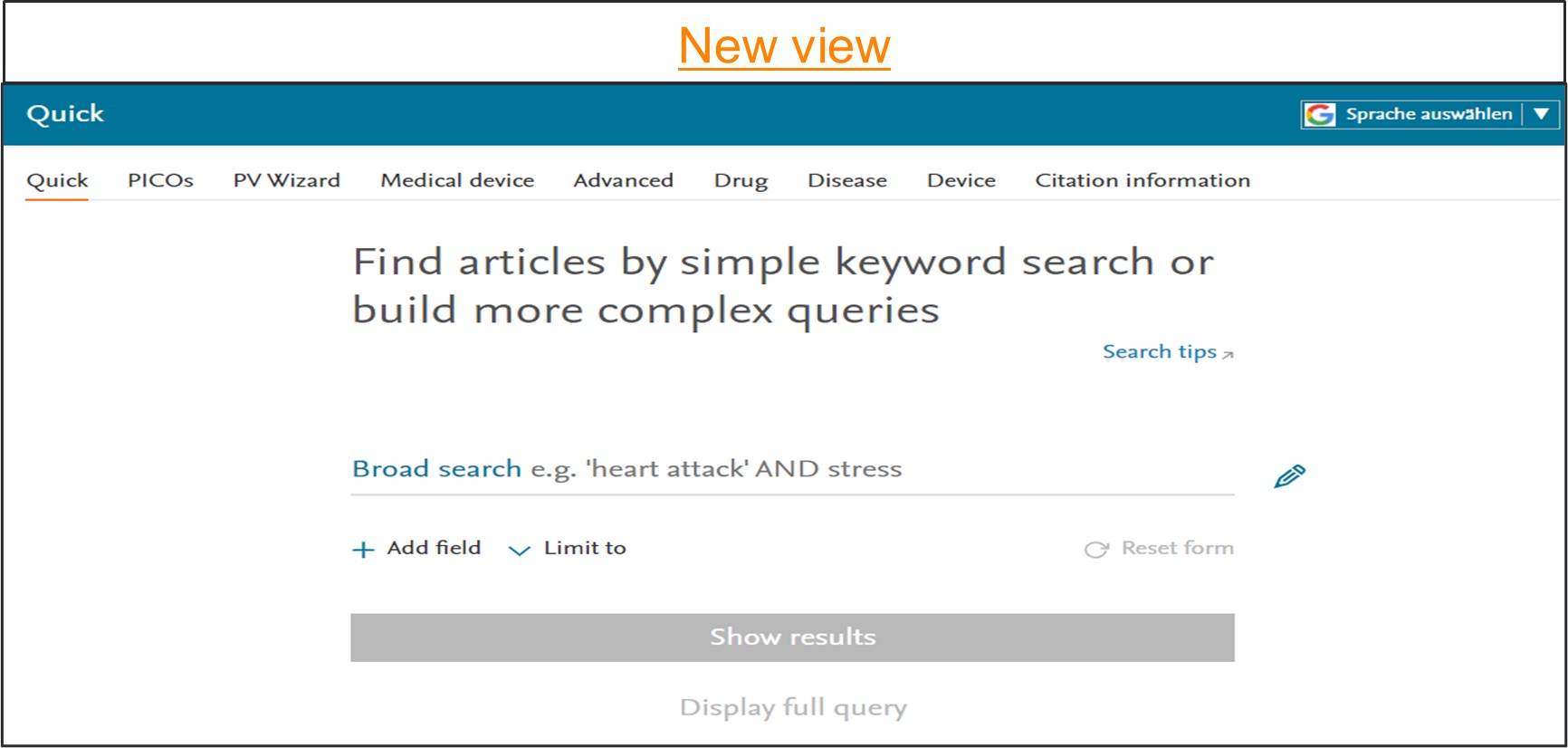
Easy to use ‘Quick search page’
We have simplified our quick search page to make it more intuitive and user friendly.
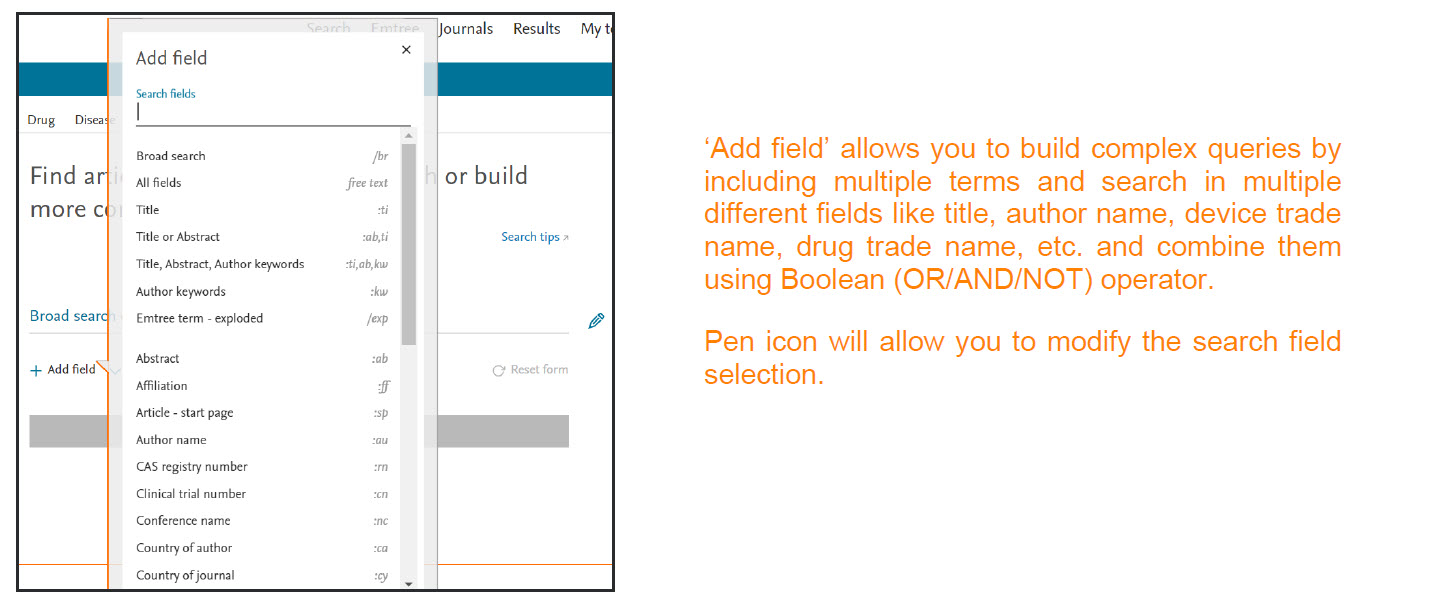
To access the Quick search, go to the homepage. In this search form you will now be presented with a single search box with an option to add multiple search fields by using the ‘Add field ‘ button.
You can add the search terms or phrase of interest into the provided search box. As soon as the term is added, Emtree will automatically suggest a preferred term that would encompass not just the entered term but also the linked synonyms. Hence using the preferred term suggested by Emtree ensures that no information is missed. On click of ‘Show results‘ button or by pressing ‘Enter‘ Embase will perform a broad search by default. It will automatically convert this to explosion plus free text search.
Improved support for ISSN field
ISSN field (:is) will now support entries with space and hyphens.
For eg. All the following variants will provide the same result hits
'10292659':is
'1029 2659':is
'1029-2659':is
Bugs
- In the Record details page –
- Full text link will work now when the DOIs are searched without using the :do field code
- Clinical trial link will work now when clinical trial number is searched without using the :cn field code
- Record with missing DOIs will not include the L1 field in the RIS export anymore.
- Loaded date field will now allow searching for following formats -
- ‘YYYY-MM’
- ‘MM-YYYY’
- ‘DD-MM-YYYY’
New and improved 'Record details page'
We have reorganized the record details page to provide you an improved user experience. This page is accessible from the search Results page which provides you a summary and highlights about the record. To view more details about a record, click on the title of the document or tick the checkbox in front of the title and click 'View'. This will bring you to the detailed view of the record. Some visual improvement made to the Record Details page are:
- Adding of Author ORCIDs details into the additional information section.
- Moved all the actionable buttons to the right-hand side of the page.
- Headings "Translated title" and "Translated abstract" will only be available for non-English articles.
- Moved author address and affiliation to the top of the screen (below title) and it will be visible only on demand.
- Highlighting of hits to make sure all the hits are immediately visible.
To know more about the record details page, click here: What can I see and do in the detailed view of a record?
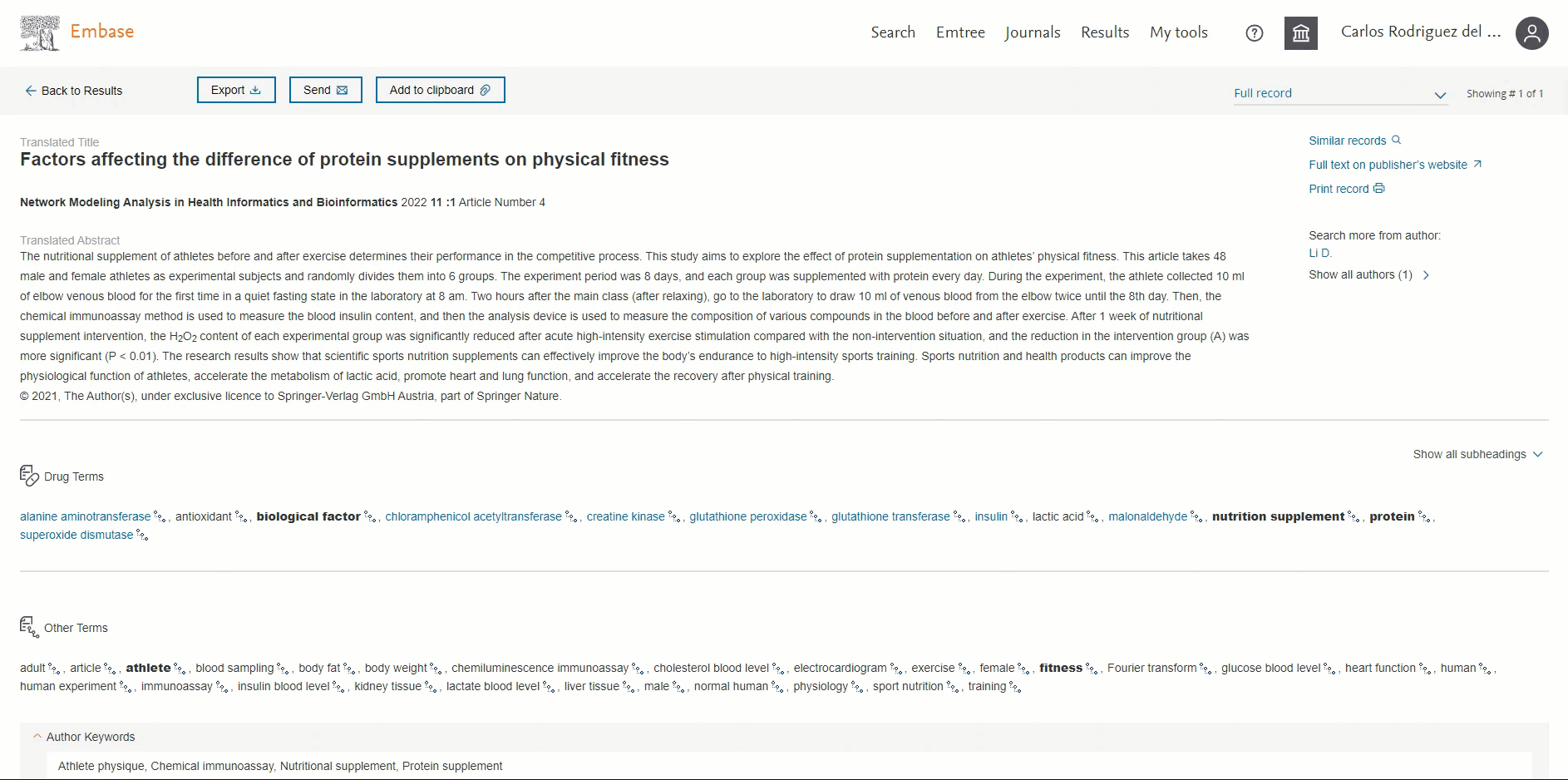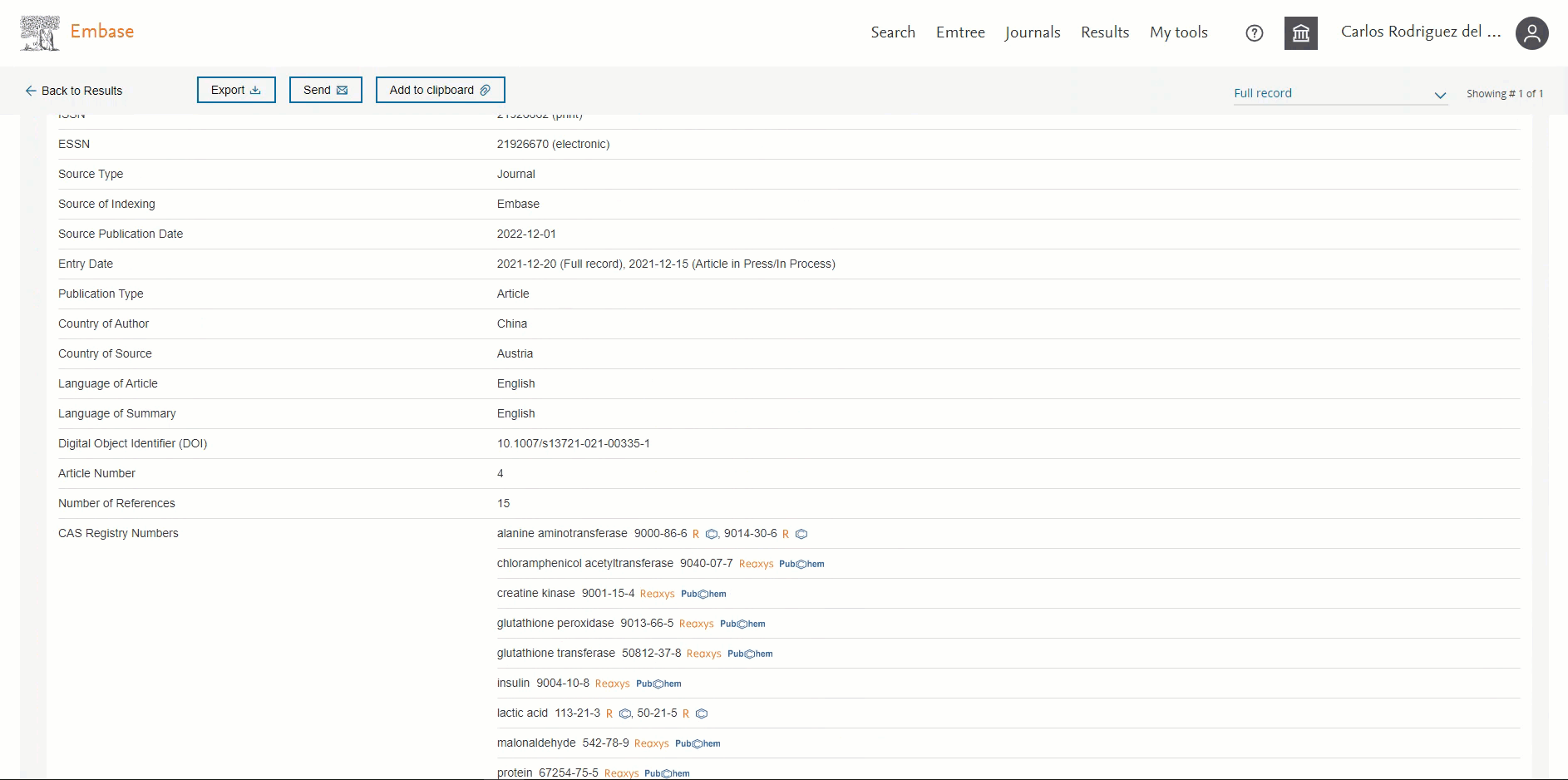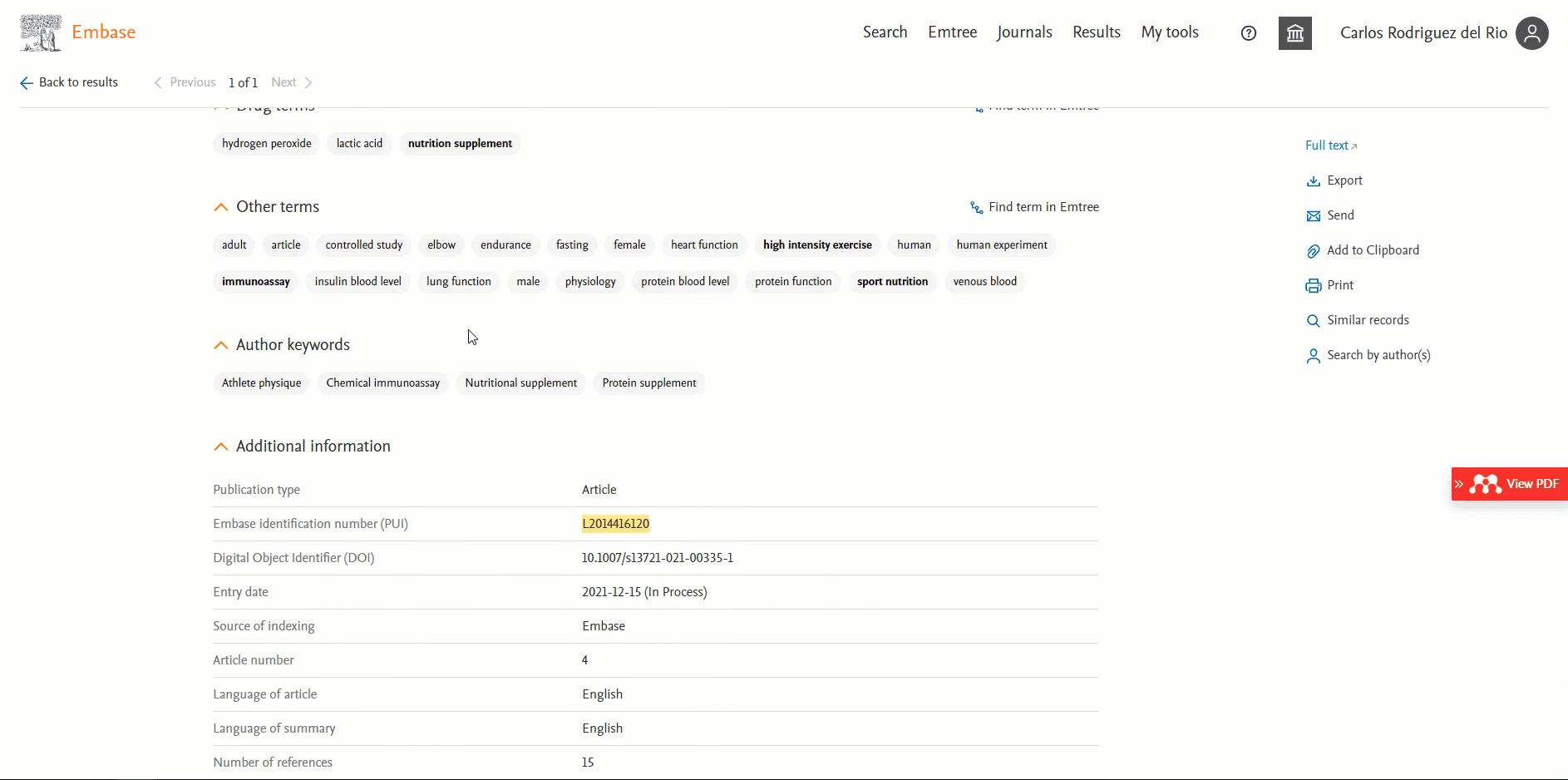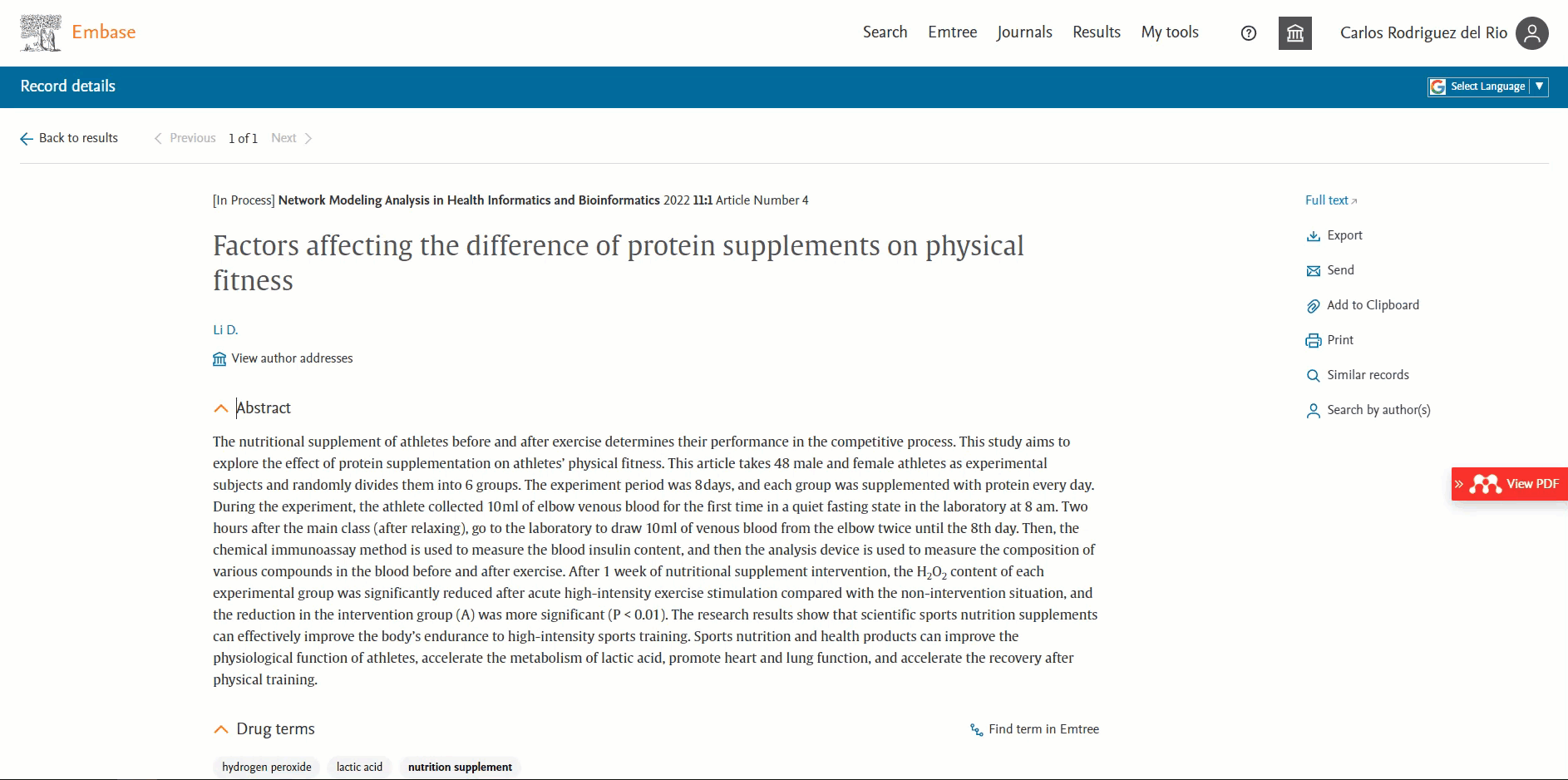
ORCID available in the 'Record details page'
Users will be able to view the ORCID of the author if present. This information will be available in the ‘Additional information’ section in the Author’s ORCIDs field. By default, the first 3 ORCIDs will be displayed; to view more you will need to click on the ‘+ # more’ button.
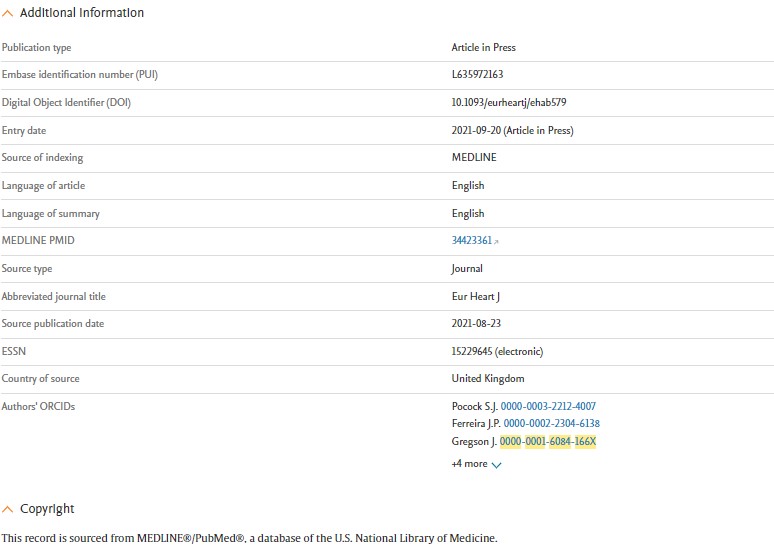
Users can now easily distinguish English vs non-English records in Embase
Headings "Translated title" and "Translated abstract" will only be shown for a non-English translated record.
The screenshot below highlights the difference between an English and a non-English record in the Record Details page:
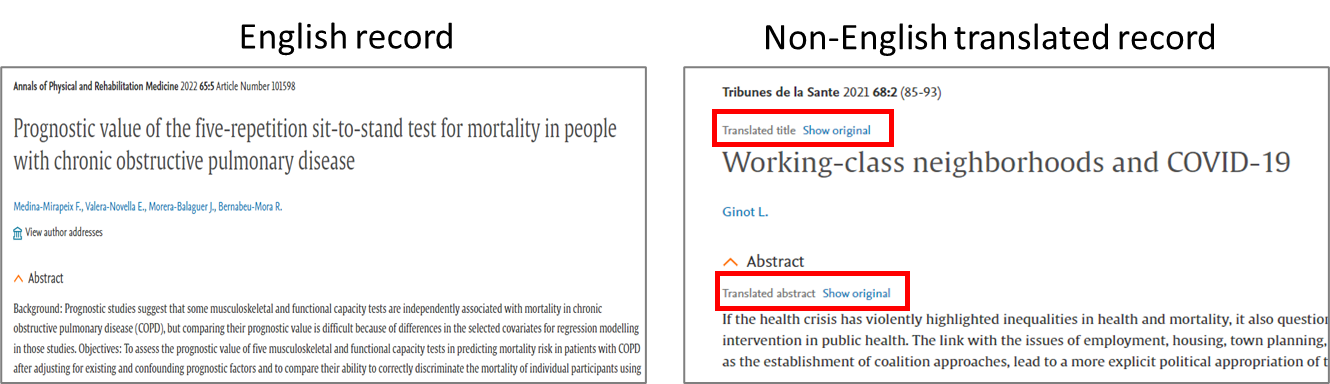
Optimized 'Preference page' and export window
In the new view we have categorized the available fields into relevant subheadings. These changes are applicable to the preference page and the export modal window. Please note that preference page in 'My tools' is only available on login. The view of all the formats have been updated - RIS format/CSV/Plain text/Pdf/MS Word/Excel/RefWorks Direct Export/ XML format
Bugs:
- In the Record details page, the article will only display Original Abstract when translated abstract is not available.
- Embase will allow combination of simple double (") or single (') quotes.
- Embase can now distinguish the Emtree terms with apostrophe (') For e.g. cytochrome c vs cytochrome c'
Did we answer your question?
Related answers
Recently viewed answers
Functionality disabled due to your cookie preferences