View by category
What are Orange Book Therapeutic Equivalence (TE) Ratings in the Drug Product Information?
Last updated on December 10, 2025
The Food and Drug Administration (FDA) evaluates whether approved products are therapeutically and pharmaceutically equivalent. Following this, products are assigned a Therapeutic Equivalence (TE) code - the first letter of the code specifies the product's rating and the second letter provides additional information from the FDA's evaluations. Sample TE codes are AA, AB, and BC.
Clinical Pharmacology reports these therapeutic equivalence ratings as designated within the Approved Drug Products with Therapeutic Equivalence Evaluationswhich is referred to as the 'Orange Book'.
The TE coding system is detailed below:
Rating | Description |
|---|---|
A | 'A' rated drugs are those the FDA deems to be therapeutically equivalent to other therapeutically equivalent products. |
AB | Actual or potential bioequivalence problems have been resolved with adequate in vivo and/or in vitro evidence supporting bioequivalence of the product to a selected Reference Listed Drug. |
AA, AN, AO, AP or AT | No in vivo bioequivalence issue is known or suspected. Designation depends on the dosage form. |
B | 'B' rated drug products are those the FDA currently considers not to be therapeutically equivalent to other pharmaceutically equivalent products. |
BC, BD, BE, BN, BP, BR, BS, BT, BX or B* | These are drug products for which actual or potential bioequivalence problems have not been resolved by adequate evidence of bioequivalence. The problem is likely to be with specific dosage forms rather than the active ingredients. |
NR (not rated) | Used for products listed in the Orange Book that are not multi-source (i.e., no FDA-approved generic equivalents). |
NA (not applicable) | Used for products that are not reviewed by the FDA, such as those marketed before 1938, vitamins, and nutritional supplements. |
Offmarket | Products designated as 'off market' include the TE code assigned, if applicable, when the product was placed off-market. |
Please note: Some drug products have more than one TE Code. Over-the-counter drugs are not assigned TE codes. Clinical Pharmacology will indicate where a TE code does not apply.
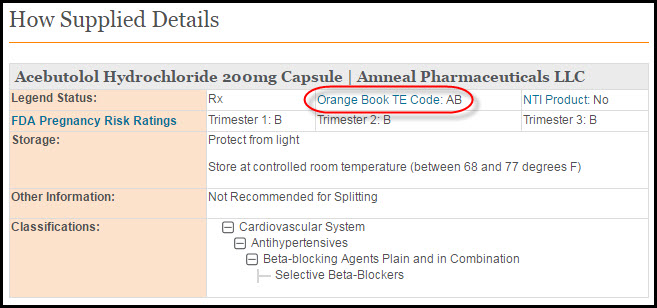
A product's TE code in Clinical Pharmacology can be found under the 'How Supplied' details of the product detail section. (The 'Orange Book TE Code' link opens a pop-up window with code definitions).
Did we answer your question?
Related answers
Recently viewed answers
Functionality disabled due to your cookie preferences