View by category
PharmaPendium Release Notes
Last updated on February 18, 2026
Addition of 19 New Safety Margin Tool Targets
Dear Valued Customer,
We are excited to announce the latest update to the Safety Margin Tool. This release expands our mechanistic safety coverage and strengthens early risk assessment capabilities.
19 new Target-MOAs (Mechanism of Action) have been added, expanding coverage across kinases, GPCRs, ion channels, transporters, nuclear receptors, and enzymes. The tool now includes 77 Target-MOAs, with a roadmap to exceed 170+ Target-MOAs by 2026, with the aim to enhance mechanistic understanding of adverse drug reactions and compound safety risks.
This release further strengthens quantitative safety margin evaluations, supporting earlier identification of off-target liabilities and more confident compound prioritization in discovery.
The Safety Margin Tool continues to integrate mechanistic pharmacology with real-world safety intelligence, enabling data-driven, evidence-based safety assessment throughout early development.
What’s coming next?
More than 90 semi-quantitative Target-MOAs will be introduced in 2026 to further expand ADR mechanistic insights across a total of 170+ targets. Continued enhancements to risk modeling and evaluation flexibility will provide even greater analytical precision and decision support.
Best regards from the PharmaPendium team:
Catherine, Sakshi, Thomas, Olivier, Ahmet, Niloufar, Jacco, Patrice, Yeliz, Abhijeet, Muthu, Mikhail, Nihit, Sathsara, Sreekantha, Aliaksandr, Branka, Jose, Igor, Arseny, Soumyadip, Boudewijn, Robert, Danielle.
Introducing the addition of FDA, EMA and ICH Guidances
Dear Valued Customer,
We are delighted to announce the latest update to PharmaPendium, introducing an important expansion to regulatory intelligence resources, including FDA, EMA and ICH guidances. These additions strengthen PharmaPendium’s role as a comprehensive reference for translational and regulatory insight into drug development and safety evaluation.
What’s New
- Unified Access across FDA, EMA and ICH Guidances:
PharmaPendium now includes full text searchable set of FDA, EMA and ICH Allowing seamless exploration alongside regulatory and scientific data. - FDA and EMA Guidances. Various categories are included for FDA and EMA guidances with a total of 1680 documents for FDA and 547 documents for EMA. These guidances aims to capture the current scientific thinking of regulatory bodies and covers topics such as Advisory Committees, Biologics, Clinical Trials. FDA contains both Draft and Final versions of guidances to ensure access to up-to-date information. For EMA apart from guidances we have also included concept papers, reflection papers, and public statements to support regulatory and scientific decision-making.
- ICH Guidances:
ICH guidances (382 documents) across efficacy, safety, and quality domains are also now available in PharmaPendium, supporting harmonized regulatory understanding across regions and simplifying best-practice alignment in preclinical and clinical development.
What’s Coming Next
In 2026, these regulatory guidances will be fully integrated into PharmaPendium AI, enabling users to query, summarize, and compare regulatory requirements using natural language.
We also plan to expand coverage to additional global agencies and introduce enhanced filtering and version tracking features.
Best regards from the PharmaPendium team:
Sakshi, Thomas, Catherine , Olivier, Ahmet, Niloufar, Jacco, Patrice, Yeliz, Abhijeet, Muthu, Mikhail, Nihit, Sathsara, Sreekantha, Aliaksandr, Branka, Jose, Igor, Arseny, Soumyadip, Boudewijn, Robert, Danielle.
Introducing Tables and Figures Extracted from FDA and EMA Approval Documents
Dear Valued Customer,
We are excited to announce the latest enhancement to PharmaPendium — the inclusion of tables and figures extracted directly from FDA and EMA approval documents. This update marks a significant milestone in expanding the platform’s depth, accuracy, and usability for researchers, regulatory experts, and safety scientists across the pharmaceutical industry.
With this enhancement, PharmaPendium now includes tables and figures from approval documents up to November 2024, encompassing data from 68,398 FDA documents and 3,045 EMA documents. These visual and tabular data sets provide immediate access to pivotal regulatory content, including study summaries, safety findings, pharmacokinetic and pharmacodynamic analyses, and efficacy results, exactly as presented in official approval documents.
Key Benefits of the New Feature
Deeper Regulatory Insights:
Researchers can now access pivotal tables and figures that summarize study outcomes, safety evaluations, pharmacokinetics, and efficacy data — as originally presented in FDA and EMA approval documents. This enables faster and more accurate interpretation of regulatory findings.
Improved Evidence Synthesis:
The structured integration of visual and tabular data allows users to correlate preclinical and clinical results more effectively, enhancing cross-study and cross-drug comparisons.
Comprehensive Assessment:
With these additions, PharmaPendium now offers an even more complete view of the drug development and approval process — combining textual review content, safety data, and graphical evidence in one integrated platform.
Time and Cost Efficiency:
Researchers and regulatory teams can locate key data points instantly, eliminating the need to manually search lengthy regulatory documents for critical tables and figures.
What’s coming next?
At Elsevier, we continue to advance PharmaPendium to support the evolving needs of scientists and regulatory professionals. The integration of visual and tabular data from FDA and EMA approval documents is another step in our mission to deliver comprehensive, evidence-based insights for safer and more effective drug development.
Best regards from the PharmaPendium team:
Sakshi, Thomas, Catherine , Olivier, Ahmet, Niloufar, Jacco, Patrice, Yeliz, Abhijeet, Muthu, Mikhail, Nihit, Sathsara, Sreekantha, Aliaksandr, Branka, Jose, Igor, Arseny, Soumyadip, Boudewijn, Robert, Danielle.
Safety Margin Tool
Dear Valued Customer,
We are excited for the latest release of the Safety Margin Tool. These new updates introduce several significant enhancements aimed at strengthening its capabilities in mechanistic safety assessment and polypharmacy analysis!
23 new Target-MOAs (Mechanism of Action) have been added with a total of 58 Target-MOAs, currently available in the Safety Margin Tool enhancing mechanistic understanding of adverse drug reactions and profiling compounds safety risks.
Users can now export results directly to Excel (Open XML format), simplifying data sharing and integration with other workflows. This new export option makes it easier to generate reports, collaborate with colleagues.
To enhance data interpretation, the tool now supports conversion of % inhibition and % stimulation values to IC50 and EC50 for values between 20% and 80% (assuming a slope of 1). This feature streamlines the process of deriving potency metrics and ensures greater consistency when comparing activity across targets and compounds.
We’ve also strengthened the link between the Safety Margin Tool and the PharmaPendium Drug Safety Dataset, enabling seamless access to clinical and FAERS data for deeper investigation at the drug, target, and adverse drug reaction (ADR) levels. This tighter integration empowers users to connect preclinical safety margins with real-world safety outcomes, supporting more comprehensive risk assessments.
In addition, users can now take advantage of an enhanced MedDRA lookup function that allows searching across the first, second, and fourth levels of preferred terms. For instance, users can easily find targets associated with terms such as hypotension, or explore results at higher levels like the vascular disorders system organ class. This makes it simpler to navigate complex adverse event data and identify safety signals of interest.
Users can now identify drugs that have been used in multiple Target-MOAs using dedicated flags to take selectivity aspects into the safety risk analysis. Overall, this release delivers a more connected, data-rich, and user-friendly experience. The Safety Margin Tool continues to evolve as a leading resource for evidence-based, mechanism-driven safety and polypharmacy evaluation.
What’s coming next?
An additional 22 Target MOA will be added early 2026 expanding ADR mechanistic insights across 170+ targets. A new risk assessment method will let users choose between Conservative and Relaxed calculation options for greater flexibility.
Best regards from the PharmaPendium team:
Catherine, Sakshi, Thomas, Olivier, Ahmet, Niloufar, Jacco, Patrice, Yeliz, Abhijeet, Muthu, Mikhail, Nihit, Sathsara, Sreekantha, Aliaksandr, Branka, Jose, Igor, Arseny, Soumyadip, Boudewijn, Robert, Danielle.
PharmaPendium AI
Dear Valued Customer,
We are excited to announce the release of PharmaPendium AI
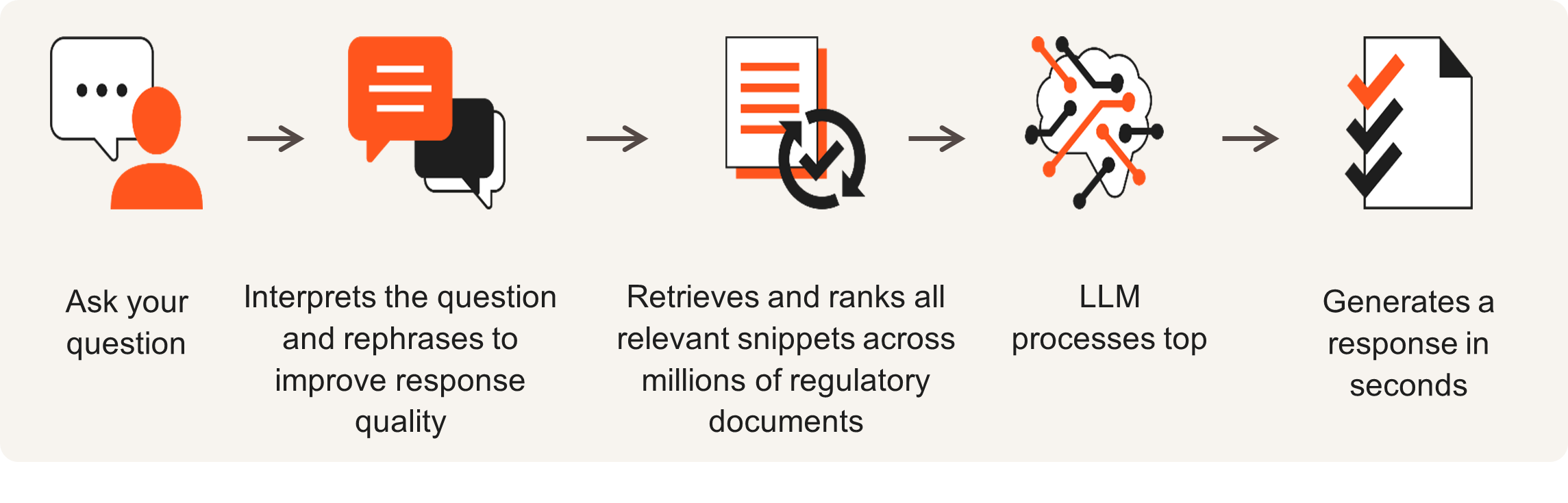
PharmaPendium AI is a natural language AI search tool on comprehensive regulatory data from the FDA and EMA. Rapid, in-depth responses surface regulatory precedents, helping regulatory affairs and R&D professionals reduce the risk of missing essential information. Optimize regulatory strategies with PharmaPendium AI.
Simplify regulatory searching with PharmaPendium AI!
Extract critical regulatory insights by leveraging natural language processing and machine learning. With this new tool, regulatory affairs, drug development and clinical research professionals can:
- Pose questions in natural language.
- Get responses from 5 million pages of verified FDA and EMA documents updated weekly.
- Link to cited research information.
- Ask follow-up questions.
- Export sessions to easily maintain records.
PharmaPendium AI-How it works:
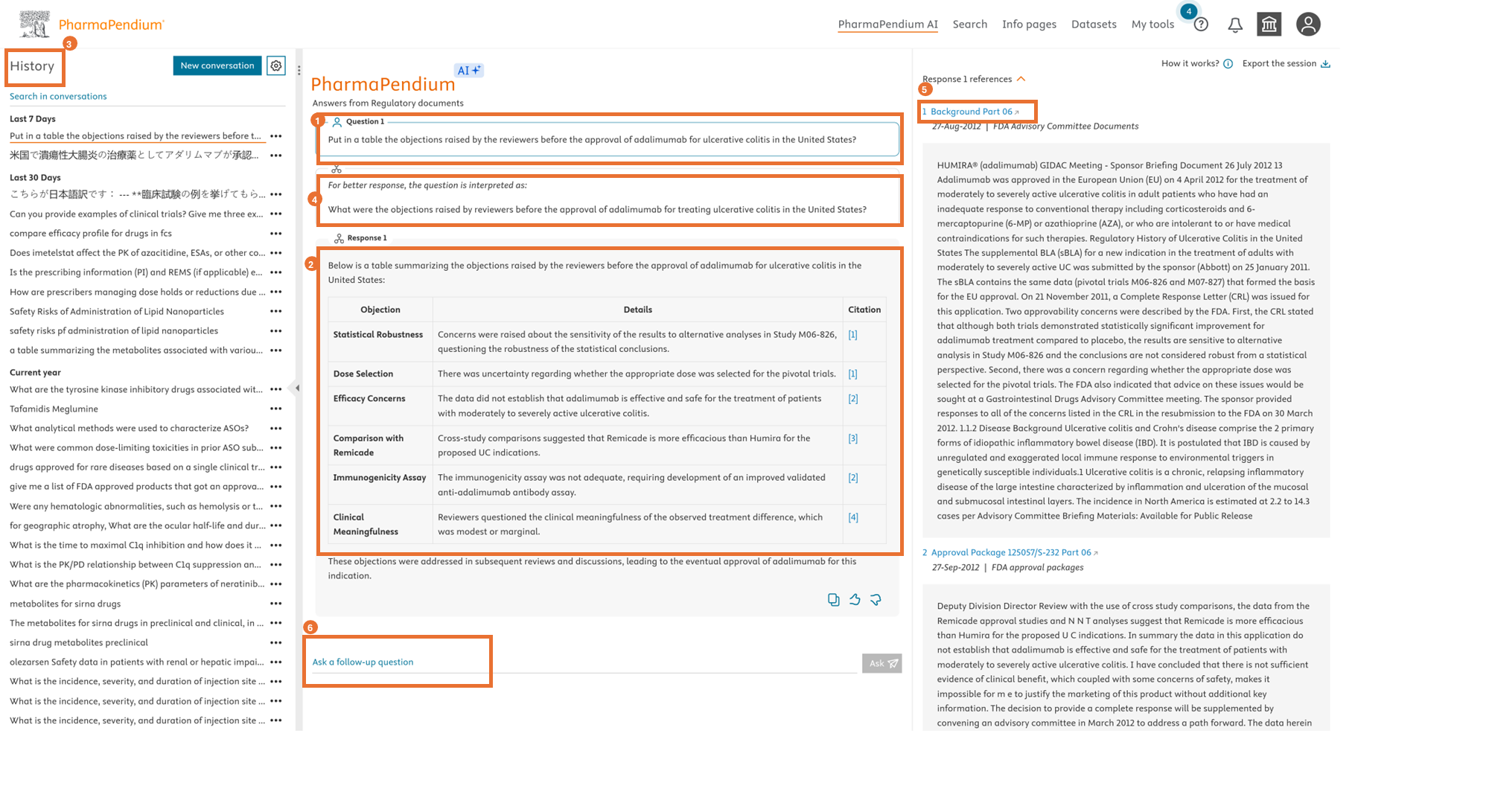
PharmaPendium AI – Enhance regulatory success - key features:
Intuitive experience:
- Ask natural language in multiple languages including English, Japanese, Korean, French, and Spanish.
- Request responses in a variety of formats including table, free text, bullet points and CSV.
- Easily search through previous responses within your history.
Transparent searching:
- Review how a question was interpreted to gain clarity and improve responses.
- Provides in-line references to allow users to dive into the original regulatory document and verify the response.
- Ask follow-up questions to refine your search and gather more regulatory insights
What’s coming next? The second phase of PharmaPendium AI is set for release in Q1 2026, with the addition of FDA, EMA, and ICH guidances.
Best regards from the PharmaPendium team:
Sakshi, Thomas, Catherine, Olivier, Patrice, Andries, Yeliz, Christian, Ahmet, Lars, Jacco, Abhijeet, Muthu, Mikhail, Willem, Khushboo, Nihit, Sreekantha, Ivan, Aliaksandr, Branka, Jose, Manasi, Nayana, Igor Arseny, Canberk, Soumyadip, Boudewijn, Karthik, Deepika, Shiva, Gorkem, Ivan, Ryan, Robert, Danielle.
Release notes, April 2025
Dear Valued Customer,
We are excited to announce the release of PharmaPendium’s Tox Navigator.
This innovative platform offers a transformative way to visualize and analyse adverse drug reactions (ADRs) across species, doses, and routes of administration, featuring unique Human Equivalent Dose (HED) curated data alongside Total Daily Dose (TDD).
Tox Navigator will enable both non-clinical and clinical toxicologists to:
Quickly visualize safety profiles across thousands of approved drugs using hundreds of thousands of calculated Human Equivalent Dose (HED) datapoints from animal doses.
- Reduce animal testing by focusing only on toxicologically relevant animals.
- Compare ADRs and their doses across various species to predict how preclinical findings might translate to humans.
- Benchmark your drug candidates against best-in-class drugs and identify critical research studies and potential risks early in the development process.
- Optimize your research efforts by focusing on candidates with favorable safety profiles, reducing the risk of late-stage failures.
Tox Navigator – Adverse events analysis - key features:
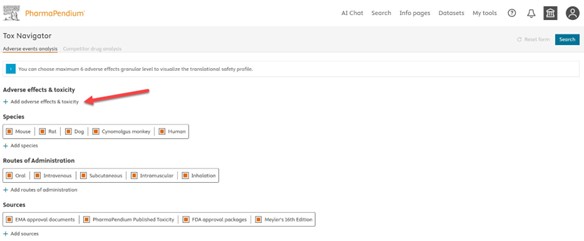
Fig.1 The starting page allows you to select up to 6 adverse effects. We have preselected by default the species, the routes of administration and all the sources but you can change and/or remove this selection should you want to.
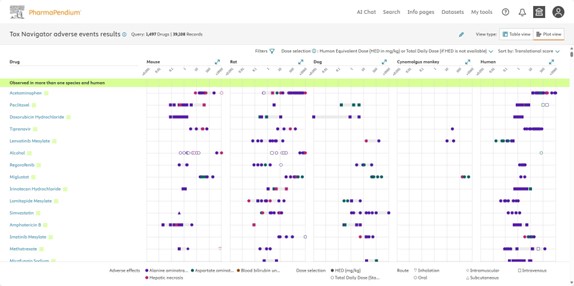
Fig.2 This plot view shows a list of drugs (1497), highlighting 4 selected adverse effects and their Human Equivalent Doses (HEDs) across various species and routes of administration, from animal studies to human outcomes.
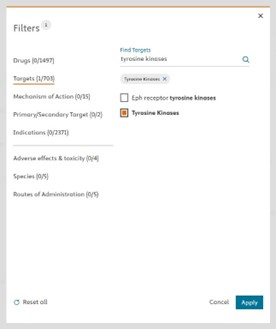
Fig.3 This filter narrows down the list of drugs by different categories (Drugs, Targets, Mechanism of Action, Indications etc…) so you can benchmark your drug candidate with the most relevant toxicity profile from approved drugs. For example, you can select drugs with “tyrosine kinases” target. (Clicking on “apply” will narrow down the drug list from 1497 to 66 drugs)
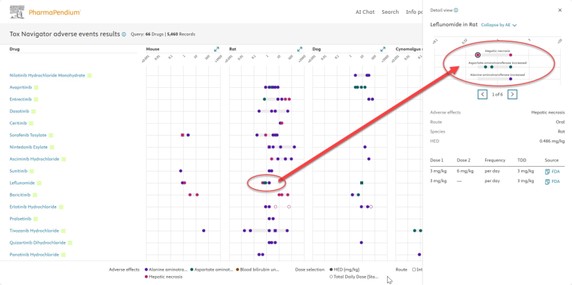
Fig. 4 In this view, the side panel displays dose extraction details, including HED, route of administration, frequency, total daily dose (TDD), and a clickable source link.
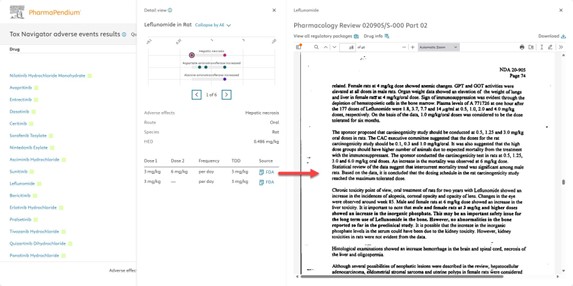
Fig. 5 When the source of information is clicked a side panel shows where the dose selection was extracted from, adding transparency and contextual information to the user flow.
What’s coming next? The second phase of the Tox Navigator is set for release in Q3 2025, enabling you to benchmark your drug candidate’s safety profile against a set of reference drugs or your preferred competitor drugs.
Best regards from the PharmaPendium team
Sakshi, Thomas, Catherine, Olivier, Patrice, Andries, Yeliz, Ahmet, Jacco, Abhijeet, Muthu, Mikhail, Willem, Khushboo, Nihit, Sreekantha, Aliaksandr, Branka, Jose, Manasi, Nayana, Igor Arseny, Canberk, Soumyadip, Boudewijn.
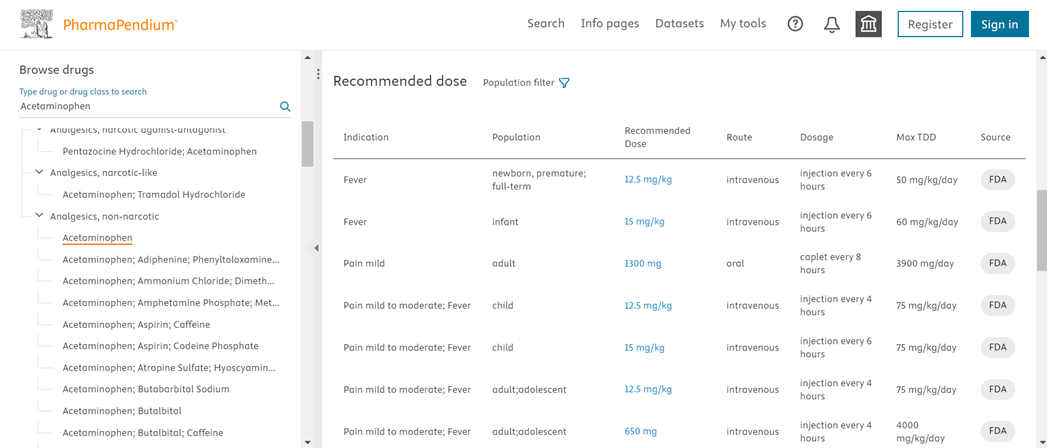
Addition of Recommended Dose Information in Drugs Info Page
Dear valued customers,
We are excited to announce our latest enhancement to PharmaPendium: the addition of Recommended Dose information in “Drugs” under “Info Pages”.
This new feature empowers you with the knowledge you need to make informed decisions throughout the drug development process. By leveraging knowledge of therapeutic doses recommended by regulatory agencies for similar drugs, you will make informed decisions, from preclinical research to commercialization, ultimately improving the chances of success for your drug candidates.
Recommended Dose information acts as a vital compass for establishing dose strategies in relation to a drug candidate. It serves several key roles:
Benchmarking: It offers a benchmark against which the dosing of a new drug can be compared, ensuring it fits within the therapeutic window established by similar, already approved drugs.
Safety and Efficacy Balance: Helps in finding a balance between the maximum therapeutic effect and the minimum risk of side effects, guiding the setting of dose ranges for clinical trials.
Competitive Differentiation: Enables comparison with similar drugs to identify potential advantages in efficacy or safety at different dosing levels, supporting strategic positioning in the market.
Risk Assessment: Assists in anticipating potential adverse events based on the dose-response relationships of similar drugs, which is crucial for regulatory approval strategies.
This information is critical for developing dosing strategies that are safe, effective, and competitive, guiding the limits of dose exploration for a new drug candidate.
Recommended Dose is available for single and combination drugs excerpted from the FDA labels and EMA annexes:
- You can access comprehensive information on the recommended doses for a particular drug across different populations, indications, and routes of administration.
- Recommended dose information has been provided for 2196 drugs until the year 2020, and the latest updates will be available soon.
Recommended Dose can be found under in Drugs under Info Pages with multiple recommendations per indication, population, route of administration.
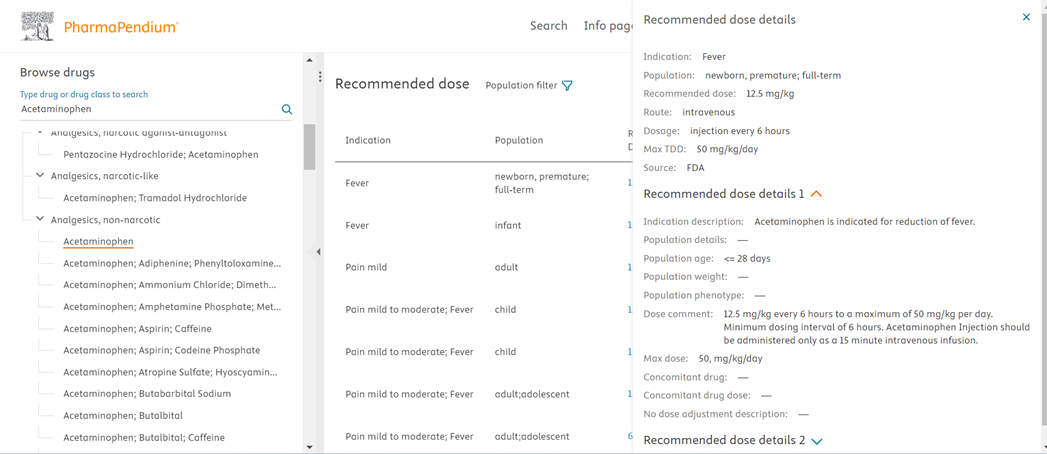
Clicking on the Recommended Dose link opens the side panel where you can find additional information associated with that recommended dose like indication description, population age, weight, dose comment, max dose which provides you comprehensive information for that dose and makes you well informed while creating a strategy for your drug candidate.
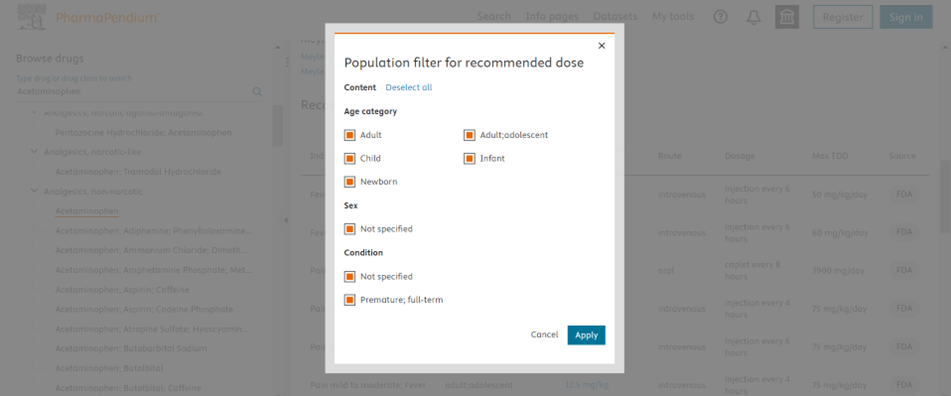
You can effectively filter the Recommended Dose data based on the population of your drug under research (Age category, Sex and Condition) resulting in a refined dataset that meets your preferences.
Exporting Recommended Dose data allows you to get all the information related to multiple recommended doses in one place.
Note: On clicking on export on Drug Info page, you export recommended dose data if its present.
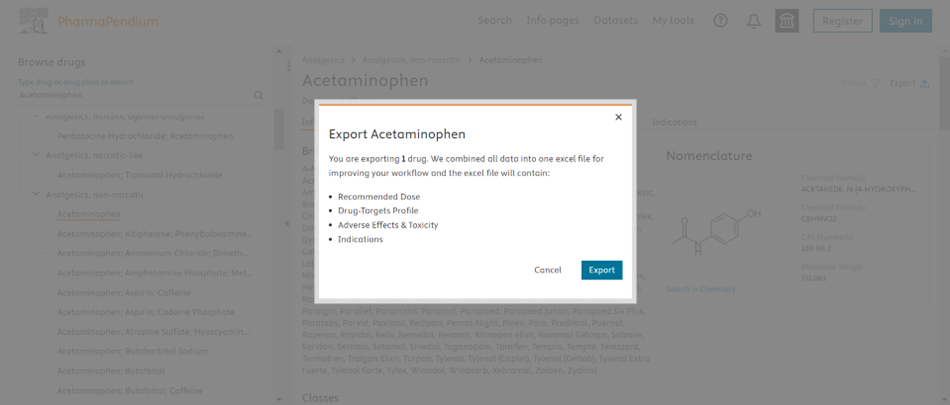
Download the pdf version of the release note here.(Opens in a new tab or window)
New Clinical Toxicity Data From Literature Is Now Available in Drug Safety
Now, your drug safety evaluations will be even more comprehensive and precise with the latest addition to Drug Safety under Datasets: clinical toxicity data from literature.
In addition to our regulatory-grade safety information, this complementary source of information will provide an unparalleled opportunity to make more informed decisions regarding the safety profile of your drug candidates.
Here's why:
- Enriched Patient Demographics and Study Group Information: Previously limited, unavailable patient demographics and study group information are now at your fingertips. By understanding factors such as age, sex, race-ethnicity, comorbidities, and genotype, you can tailor your safety assessments to specific patient populations.
- In-depth Adverse Effects Insights: Obtain detailed information on adverse effects, including severity, specific details, case numbers, and event timing. This facilitates quicker and more effective risk mitigation, improving patient safety during drug development.
- Easy Access to Essential Data: You will find all the relevant data points for your drug safety assessment conveniently available in one place by clicking on the View Full Study.
- High-Quality Clinical Literature Coverage: Our clinical toxicity data is derived from high-quality clinical literature spanning from 2019 to 2023, covering 1210 drugs and 9714 articles. 2024 articles will come soon.
New columns added for clinical toxicity:
# | Column name | Category | Before | Now |
|---|---|---|---|---|
1 | #N (total number of cases) | Results | ❌ | ✅ |
2 | Study Number | Study Information | ❌ | ✅ |
These new fields can be customized in the Show/Hide columns options in the Drug Safety module as well as in the Export feature of the module.
For any feedback or questions, reach out to us through any of our contact channels below.
Thank you for choosing PharmaPendium.
Best regards from the PharmaPendium team
Sakshi, Thomas, Olivier, Patrice, Andries, Yeliz, Igor, Ahmet, Jacco, Abhijeet, Muthu, Mikhail, Willem, Khushboo, Nihit, Sreekantha, Aliaksandr, Branka, Jose, Iaroslav, Arseny, Canberk, Soumyadip, Boudewijn
Download the pdf version of the release note here.(Opens in a new tab or window)
The New PharmaPendium now has improved Autocomplete and export functions
Dear valued customers,
We are excited to announce the release of new features in PharmaPendium that will significantly enhance your user experience. This update introduces Autocomplete Phase 2 for faster information retrieval and the possibility to export visual overview in an Excel spreadsheet (PNG format). We are confident that these improvements will further streamline your research process and empower you to make more informed decisions.
Autocomplete Phase 2 for faster retrieval
- Autocomplete Phase 2: Building on the success of our intelligent autocomplete feature, Phase 2 brings even faster information retrieval. This enhancement enables you to narrow down your search results with greater precision and efficiency, making it easier than ever to access critical regulatory-grade information.
- Drug/drug class, Target/target class, Indication/indication class + free text: See example below: Selection is all drugs in the “antivirals” class with “rabbit” in the document result page. Note that the possibility is given to search through all subscribed datasets with the drug class antivirals only below the grey line.
- Free text + Free text: See example below: Selection is “cardiac disorders” AND “rapidly progressive” within the same snippet in the document result page.
- Drug/drug class, Target/target class, Indication/indication class + Adverse effects: See example below: Selection all drugs by “5-HT-1 Receptors” target class AND adverse effects are “abdominal discomfort” leading to the drug safety datasets.
If the drug, target or indication - Adverse effects association find no result the search button is deactivated.
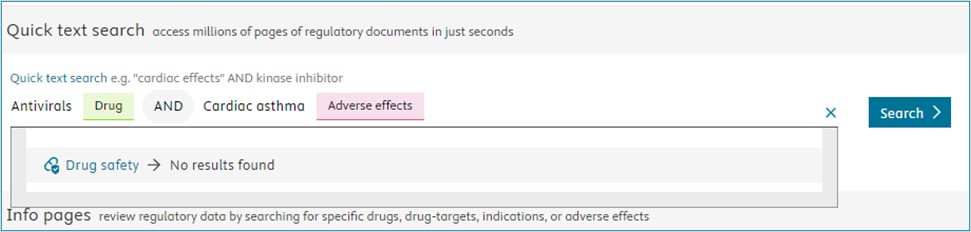
Export of visuals now possible
- Visual Summary Export in an Excel spreadsheet (PNG Format): As a separate feature, we have added the option to export visual summaries as PNG files in a new tab. This functionality allows you to easily include PharmaPendium's best-in-class visualizations in your reports, presentations, and other documents, providing more flexibility in sharing and presenting your findings.
After exporting the file*, check the Visual Overview tab below:
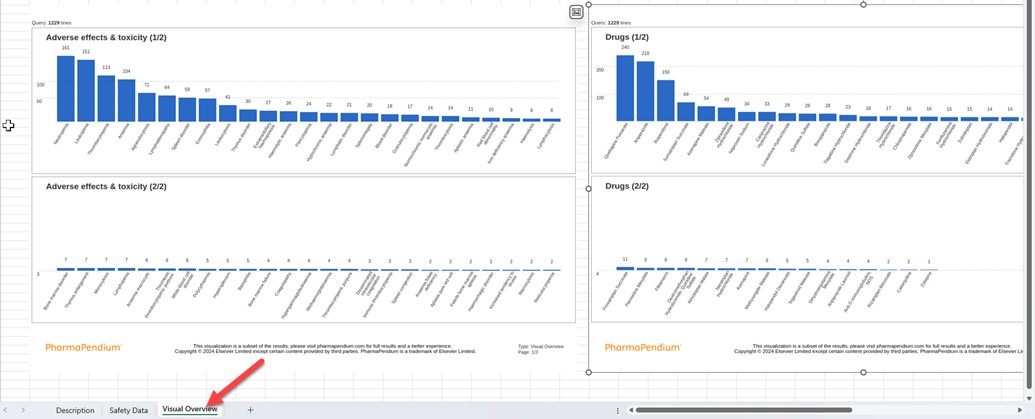
*In this release phase, for the export Excel, up to a maximum of 50 graph items per category will be shown.
These new features, combined with the cutting-edge user interface and streamlined navigation introduced in the previous release, make PharmaPendium an indispensable tool for pharmaceutical professionals.
For any feedback or questions, reach out to us through any of our contact channels below.
We are committed to improving your user experience and providing you with the tools you need to succeed in the world of pharmaceutical research.
Thank you for choosing PharmaPendium.
Best regards from the team
Thomas, Sakshi, Olivier, Ahmet, Jacco, Abhijeet, Muthu, Mikhail, Willem, Khushboo, Raj, Sreekantha, Jose, Branka, Mani, Igor, Arseny, Canberk, Mallick, Boudewijn
Download the pdf version of the release note here.(Opens in a new tab or window)
The New PharmaPendium provides a complete translational view including demo versions from Nov 2nd, 2023
Dear valued customers,
We are excited to announce a significant update as part of the new PharmaPendium that empowers all users with broader access to our extensive datasets. With this release, we make it possible for all of you to explore features of the datasets you have never seen before. Learn about the new PharmaPendium in this short video.
Enhanced translational experience in demo view at no additional cost
- Truly translational: Search across all PharmaPendium datasets
- Non-subscribed datasets (demo ribbon) will show a limited demo view
- You will be able to view a maximum of five lines of data from each dataset
- Unlimited filter functionalities for five lines
- The filters are applied on the whole dataset, and only the first five lines are shown depending on the filter
- The filters are not restricted to only five lines of results
FAERS Viz is now prominently featured within the category Predictive Tools
- The FDA Adverse Event Report System, now referred to as FAERS Viz, helps you find any adverse event from real-world evidence and post-market drug safety faster
- Assess how adverse events for a single drug compare with its drug class
- Understand the prevalence of co-morbidities not seen during clinical trials
- Get critical insights on drug-drug interactions (DDIs) that become evident post-market
- At no additional cost - part of the basic subscription
Let's have a look:
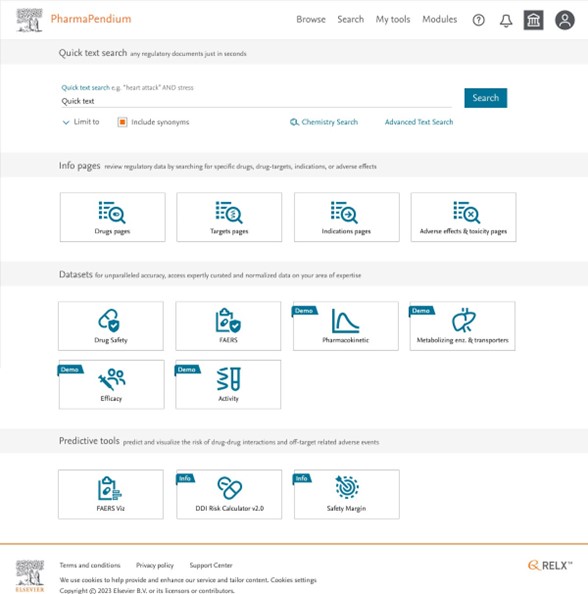
Example of a basic subscription. Non-subscribed parts are shown labeled “demo” for datasets and “info” for predictive tools.
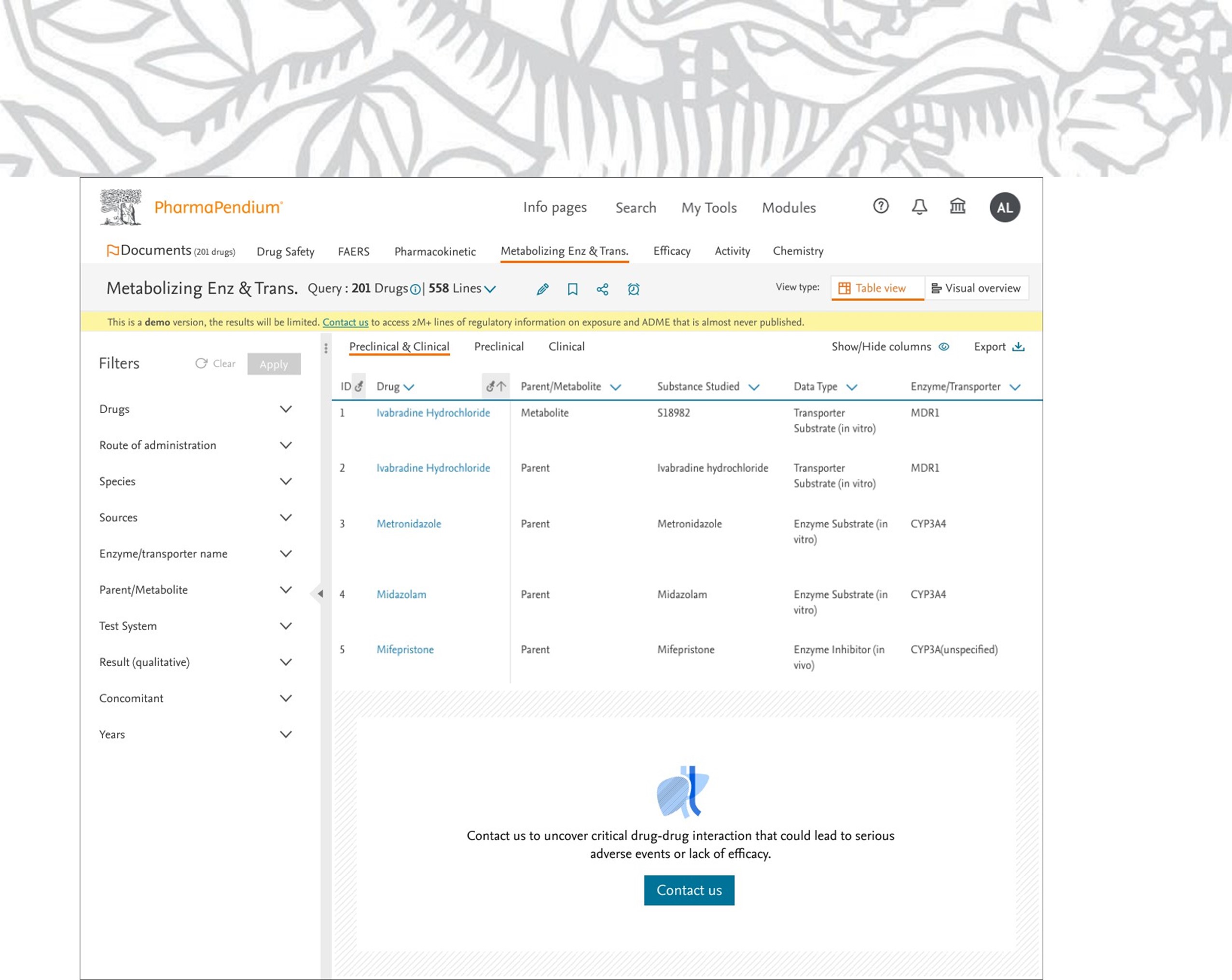
Five lines of search results are shown within the demo version of a non-subscribed dataset, in this example for Metabolizing Enzyme and Transporter dataset (MET).
For any feedback or questions, reach out to us through any of our contact channels below.
We are committed to improving your user experience and providing you with the tools you need to succeed in the world of pharmaceutical research.
Thank you for choosing PharmaPendium.
Best regards from the team
Thomas, Sakshi, Olivier, Ahmet, Jacco, Abhijeet, Muthu, Mikhail, Willem, Khushboo, Raj, Sreekantha, Aliaksandr, Branka, Jose, Igor, Iaroslav, Arseny, Canberk, Mallick, Boudewijn
Download the pdf version of the release note here.(Opens in a new tab or window)
The New PharmaPendium: Elevating Data Accessibility to Unprecedented Heights with an Enhanced, Intelligent User Interface
Dear valued customers,
Welcome to the New PharmaPendium with a translational view. We are thrilled to introduce the cutting-edge user interface (UI) that sets new standards for user experience and data accessibility.
PharmaPendium's new, intelligent UI is a game-changer for pharmaceutical professionals. It simplifies your research process, improves data digestion, and empowers you to make more informed decisions in a dynamic and data- intensive field.
In combination with our best-in-class visualizations and intelligent autocomplete, accessing critical regulatory-grade information has never been so easy.
How the new interface helps you find answers faster
- Streamlined, user-friendly navigation: Our tab-style display organizes data into logical categories, eliminating the need to navigate through multiple siloed result pages.
- Say goodbye to information overload: Each dataset that can be evaluated and processed via either a visual or table, making it easier to process and absorb information effectively.
- Enhanced visualization: Enjoy interactive charts, graphs, and visual aids that simplify complex data
- Faster access to relevant information: Switch between different types of information or result sets with a single click, saving valuable time and improving efficiency.
Let’s have a look:
Example: My drug candidate targets the “calcitonin gene-related peptide receptor” I need to know:
- The toxicity profile of approved drugs which target the same receptor.
Just type “cal” and scroll down and click on your target of interest:
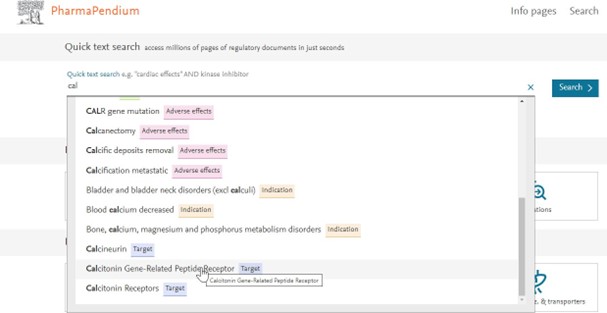
Dive into the Drug Safety Dataset by selecting your target of interest “calcitonin gene-related peptide receptor”:
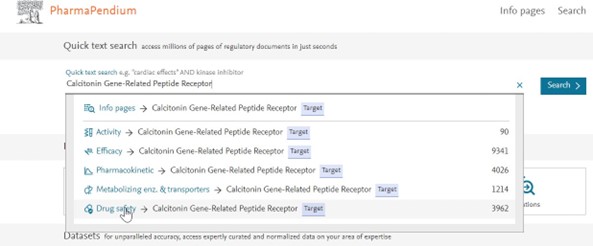
Check our new Tab-style user interface where you can easily see and access other information of interest* (FAERS, PK, MET, Efficacy, Chemistry etc…):
*subject to subscription level
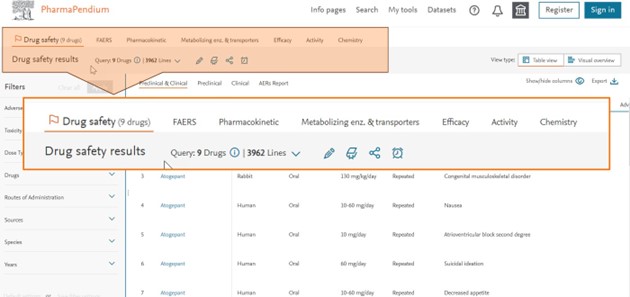
You can easily filter your information with our new intelligent visual overview:
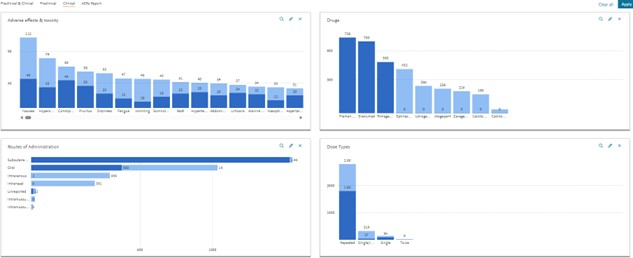
Dive into other information of interest, like the pharmacokinetic dataset and select the graphs you are interested in (e.g. select AUC, Cmax and T1/2):
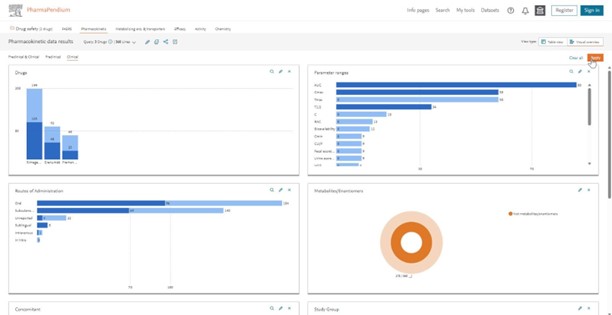
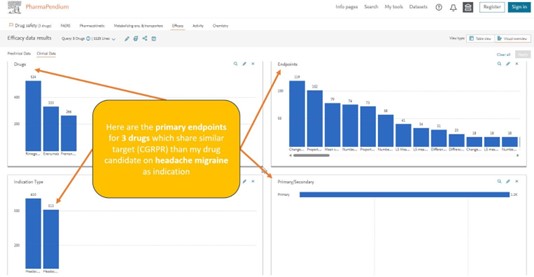
You can now easily move to another area of interest like the efficacy dataset to better understand for example the primary endpoint selection for your drug candidate who share the same target and indication:
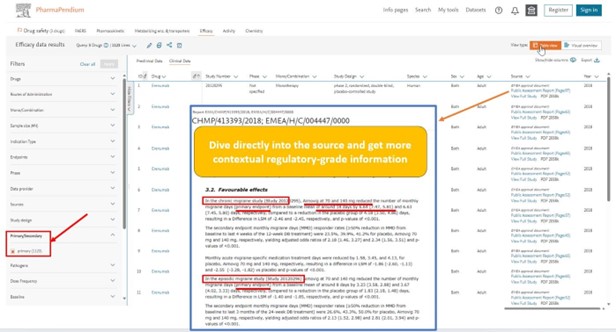
You now can come back to the table view and dive directly in the source of document where we have extracted hundreds of data point:
For any feedback or questions, reach out to us through any of our contact channels below.
We are committed to improving your user experience and providing you with the tools you need to succeed in the world of pharmaceutical research.
Thank you for choosing PharmaPendium.
Best regards from the team
Thomas, Sakshi, Olivier, Ahmet, Jacco, Abhijeet, Muthu, Mikhail, Willem, Khushboo, Nihit, Sreekantha, Aliaksandr, Branka, Jose, Igor, Iaroslav, Arseny, Canberk, Soumyadip, Boudewijn
Download the pdf version of the release note here.(Opens in a new tab or window)
Empowering Research and Regulatory Intelligence with New Search and Visual Capabilities
Dear valued customers,
Extracting meaningful insights from vast amounts of complex data is a challenge. Traditional methods of data analysis can be time-consuming, cumbersome, and may not reveal intricate patterns that can impact crucial decisions.
PharmaPendium revolutionizes the way researchers and drug developers extract valuable insights. With the introduction of two powerful features, Autocomplete and Dynamic Visual Summaries, PharmaPendium is unlocking new possibilities for comprehensive data analysis, uncovering hidden patterns, and facilitating informed decision-making.
Enhanced Efficiency and Productivity: Autocomplete will accelerate your search process
Autocomplete enables you to rapidly explore the vast database with ease and accuracy. By intelligently suggesting terms and related concepts as users’ type, autocomplete accelerates the search process, saving valuable time and ensuring more accurate and efficient data retrieval.
You can swiftly navigate through drug names, targets, adverse effects, indication and more, streamlining their workflow and enhancing productivity without the risk of missing critical regulatory-grade information that can make a difference in the long journey to drug development.
Autocomplete reduces search time and simplifies the data retrieval process, enabling you to focus more on analysis and interpretation. This streamlined workflow enhances productivity and accelerates research timelines.
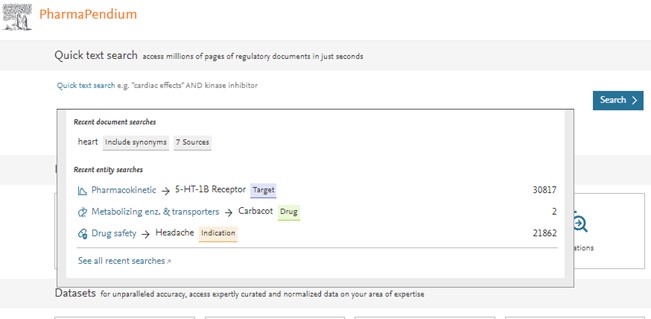
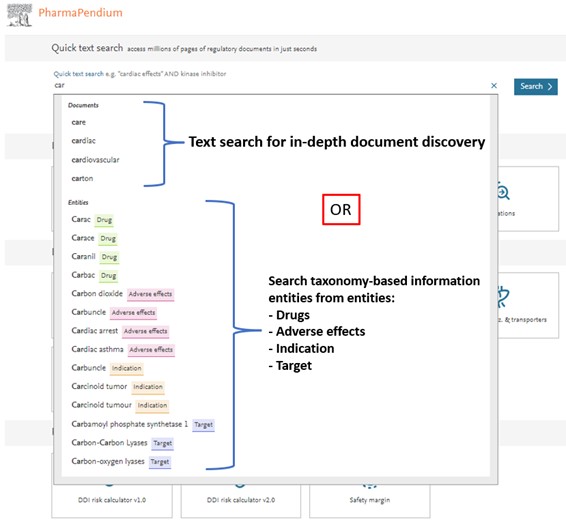
- Find your recent searches in one click to save time:
- Navigate through our documents or our main entities like drugs, targets, adverse effects or indications of interest:
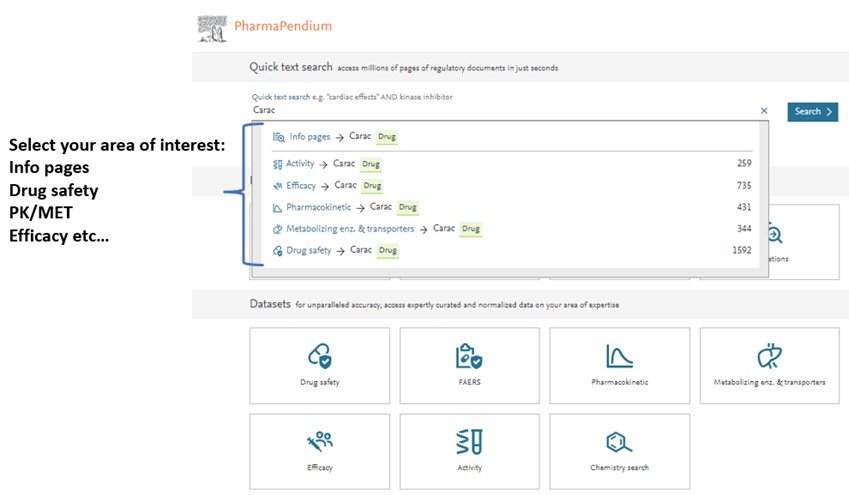
- Dive into your area of interest once you have selected your preferred choice, a drug or drug class, target or target class, adverse effects, or indication:
Dynamic Visual Summaries: Uncovering Insights and Patterns for Improved Decision-Making
The new feature, Dynamic Visual Summaries, across all areas of interest (drug safety, PK, MET, efficacy, chemistry, and activity datasets) provides you with an interactive way to analyze data.
Dynamic Visual Summaries transform complex data into visually appealing representations, facilitating quick identification of trends, outliers, and relationships. You will grasp the bigger picture effortlessly and make informed decisions based on comprehensive data analysis.
By dynamically generating graphical representations based on main parameters, such as efficacy, safety, pharmacokinetics, and toxicity, you will gain a comprehensive overview of the dataset immediately.
Through easy-to-use filtering options, these visual summaries adapt in real-time, allowing you to explore patterns, trends, and correlations that might otherwise remain hidden in tabular data. The ability to visualize data empowers you to make data-driven decisions with greater confidence.
With access to powerful visualization tools, you will gain deeper insights into drug efficacy, safety, and other critical parameters. This empowers you to identify potential risks, optimize drug development strategies, and support evidence-based decision-making throughout the entire product lifecycle.
How to use the new Dynamic Visual Summary feature:
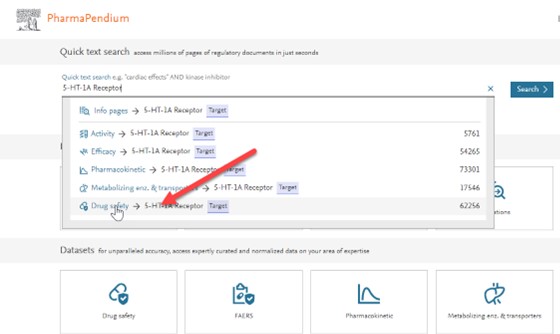
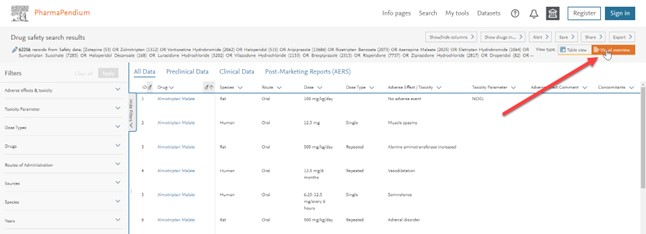
- Type '5 -HT-1A' on entities then click on the drug safety dataset:
- When you land on results page click on the upper right 'Visual overview' button:
You can now check our visual overview page
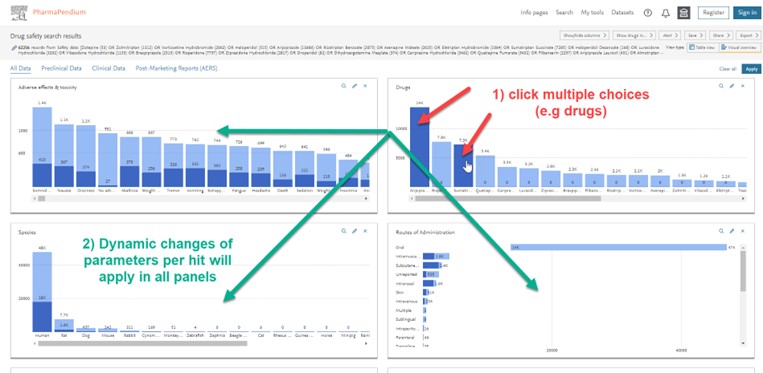
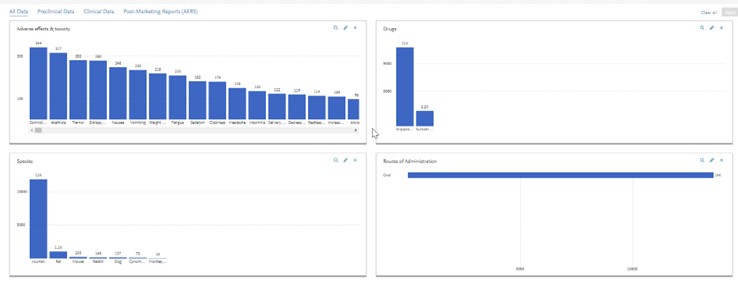
- You can click on various graphs/parameters and see the dynamic changes live in all other panels
- Click apply and only the data selected will appear:
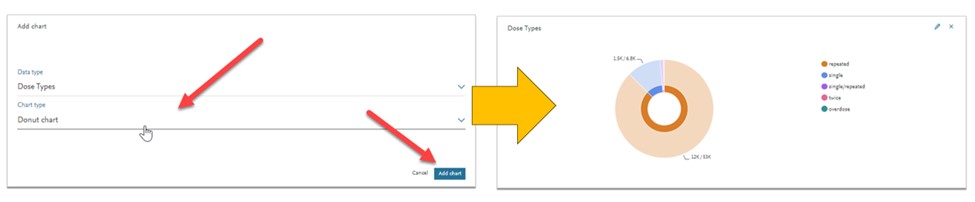
- You can add the parameters and the chart display of your choice:
By introducing Autocomplete and Dynamic Visual Summaries, PharmaPendium demonstrates its commitment to driving innovation and empowering you with advanced tools for data analysis.
This is a big step in the journey to the New PharmaPendium in Q4 2023.
We look forward to providing you with even more innovative features in the future.
For any feedback or questions, reach out to us through any of our contact channels below.
Best regards from the team:
Thomas, Sakshi, Olivier, Ahmet, Jacco, Abhijeet, Vitaly, Shweta, Mikhail, Willem, Khushboo, Sreekantha, Aliaksandr, Branka, Jose, Igor, Iaroslav, Arseny, Canberk, Shakhriyor, Boudewijn
Download the full release notes here.(Opens in a new tab or window)
New homepage and new PDF viewer for faster access to drug information
Dear valued customers,
We are thrilled to announce the latest update to PharmaPendium, which includes several new and exciting features designed to make your work more efficient and effective.
New homepage for more convenient access
Our new homepage will make it more convenient for you to access critical information around drugs, targets, indications, and drug-target-mechanism of action relationships for a quicker and more accurate toxicity profiling for your drug candidates.
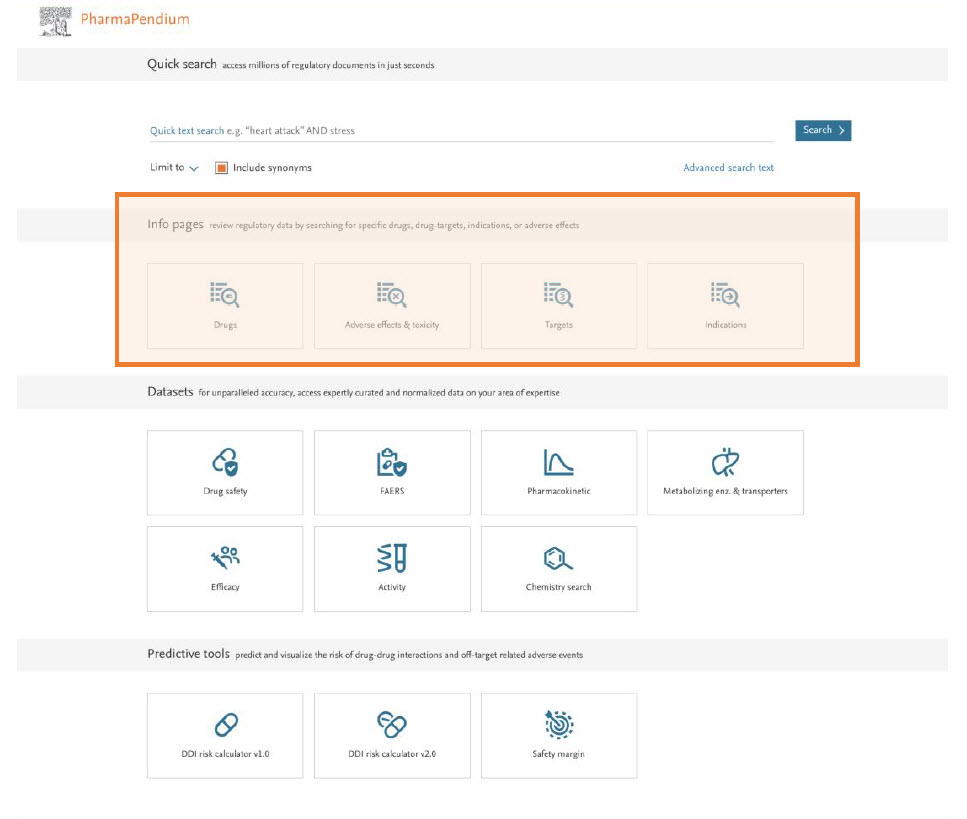
Moreover, this new homepage will allow you to make more accurate requests directly from your sources of choices (e.g., select EMA and FDA only) or select the years of interest (e.g., 2011 to 2021).
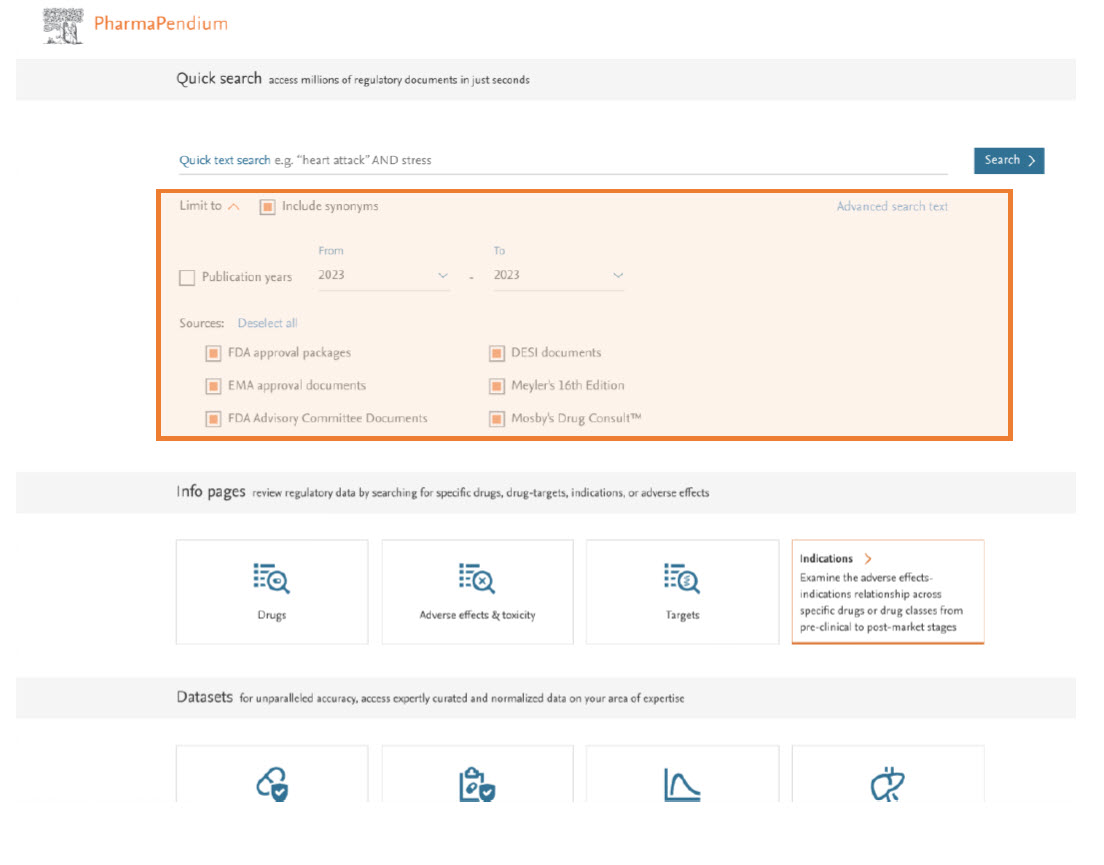
Upgrade of all the information pages including a new, enhanced pdf viewer
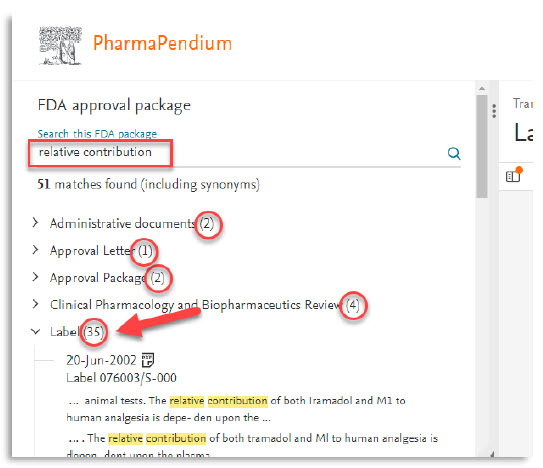
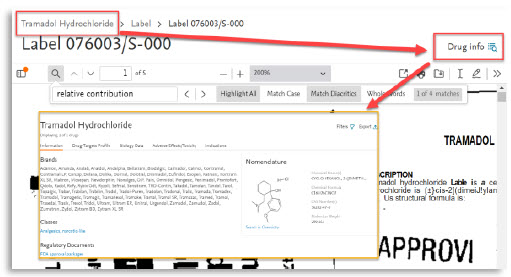
- The upgraded pages offer enhanced search and function capabilities such as the ability to see the numbers of searched terms by subcategories of approval documents. These pages also include a direct link to the drug of interest.
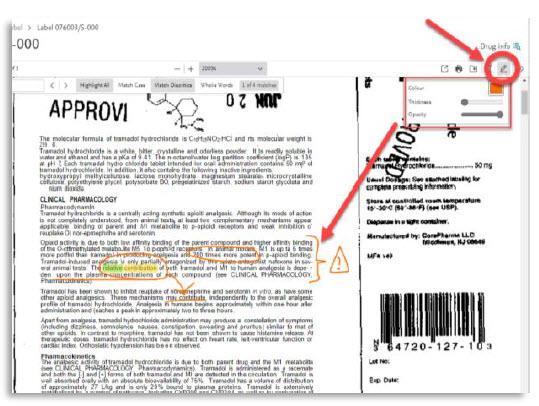
- The new pdf viewer will allow you to search for multiple terms simultaneously, making it easier and quicker to find the relevant information. In addition, you can now add annotations and drawings directly to the PDF, allowing you to highlight and mark up important information for later reference.
Time-saving and increased efficiency for regulatory affairs professionals
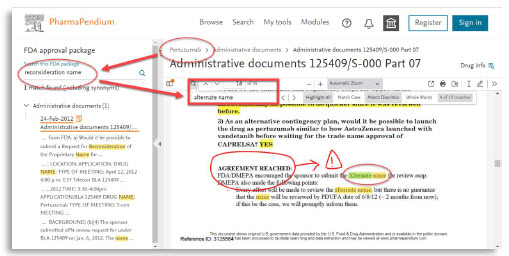
- “When was a proprietary name submitted for consideration and denied?”
- “How was this issue discussed with regulatory agencies?”
Regulatory affairs professionals who often need to review complex regulatory documents and search for multiple terms at once, can now quickly locate specific pieces of information within the document, which saves time and increases efficiency:
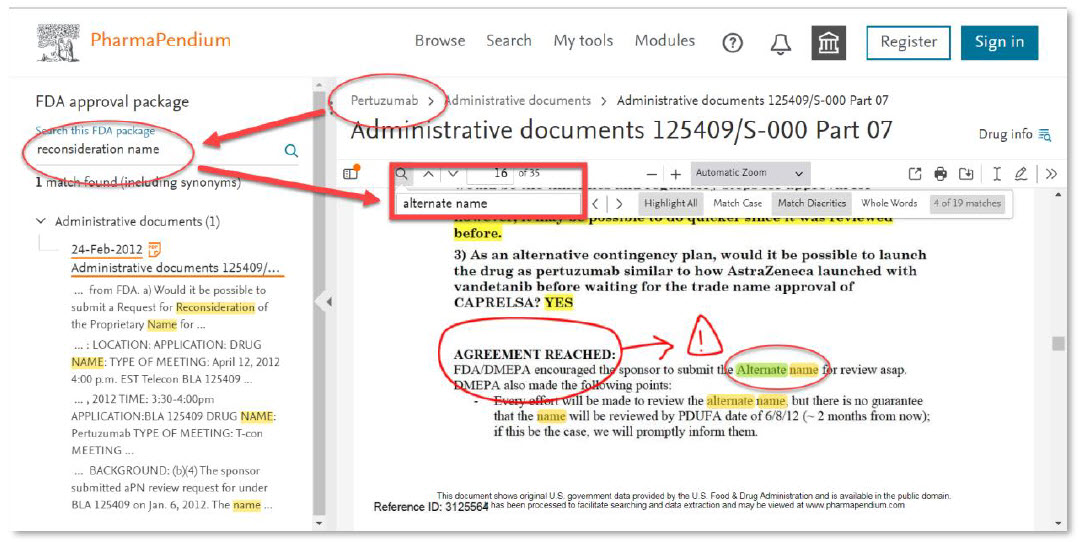
Now, the use of double terms “Alternate name” can be retrieved for rapid and accurate evaluation. Some annotations can also be done to highlight the matter.
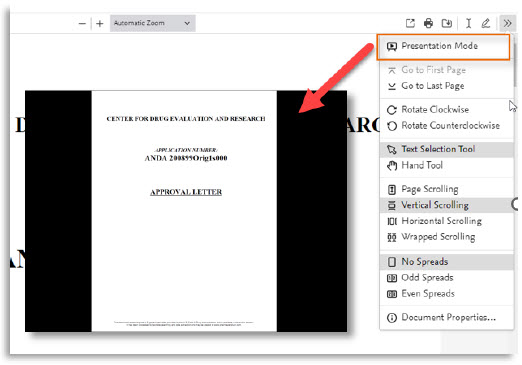
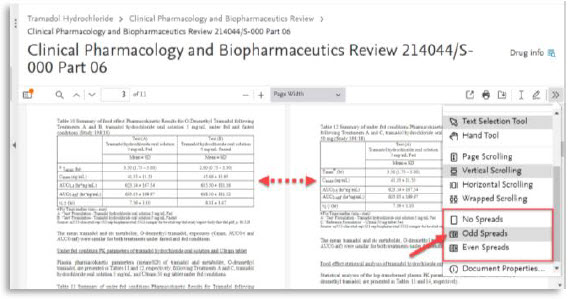
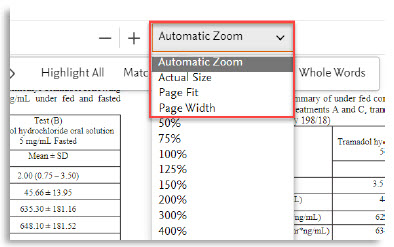
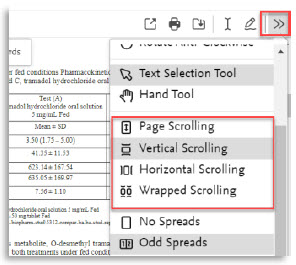
Here are all the new features of the PDF viewer:
# | Feature name | Visual |
|---|---|---|
1. | Number of hits per subcategories |
|
2. | Drug info link |
|
3. | Search with multiple terms |
|
4. | Rotation of documents |
|
5. | Presentation mode |
|
6. | Spread view both add and even: > to compare side by side tables or figures |
|
7. | Annotation with text and drawing |
|
8. | Convenient and automatic zoom modes |
|
9. | Document properties |
|
10. | Different options for page scrolling |
|
11. | Tablet friendly |
|
We believe that these new features will significantly improve your experience with PharmaPendium and help you to perform your work more efficiently and effectively.
We look forward to providing you with even more innovative features in the future.
For any feedback or questions, reach out to us through any of our contact channels below.
Best regards from the team:
Thomas, Sakshi, Olivier, Ahmet, Jacco, Abhijeet, Vitaly, Shweta, Mikhail, Willem, Khushboo, Sreekantha, Aliaksandr, Branka, Jose, Igor, Iaroslav, Arseny, Canberk, Shakhriyor, Boudewijn
Download the pdf version of the release note here(Opens in a new tab or window)
New Browse Pages with increased dynamic filter capabilities and readability
- New user-friendly interface for easier safety profiling and reading experience.
- New dynamic filter capabilities will help benchmarking your drug candidate on the most relevant approved drugs that share the same toxicity profile.
- Unified export so you can have all information on relevant filter-drug selection.
New user-friendly interface with better layout:
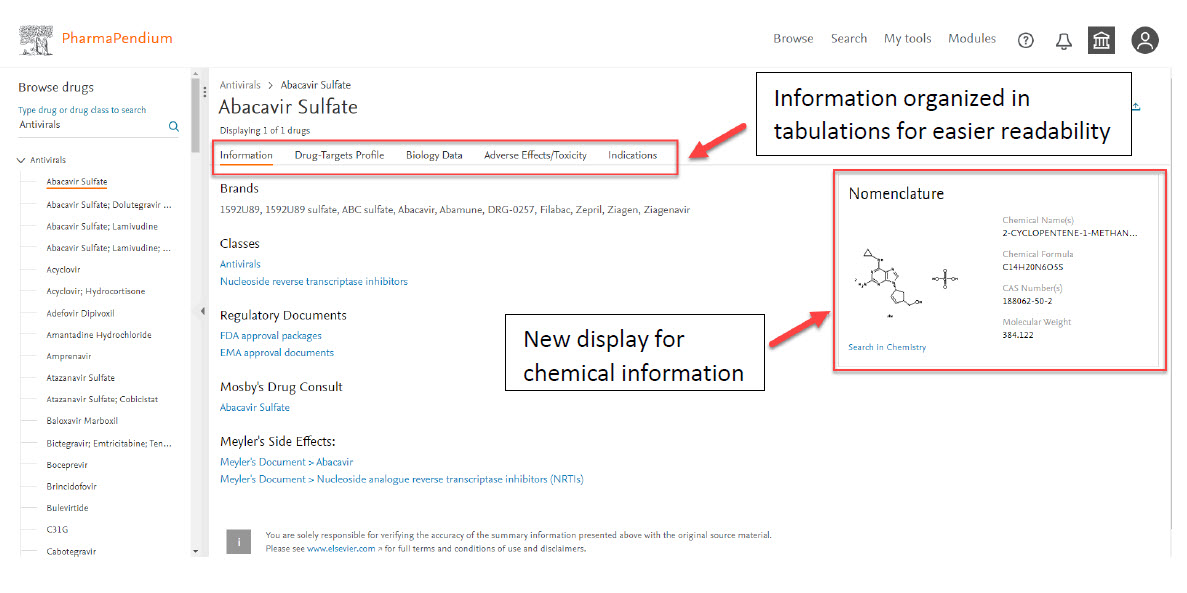
New dynamic filter capabilities will help benchmarking your drug candidate for higher accuracy on toxicity profile.
Now you can quickly answer the following questions:
- What is the translational safety profile for drugs which like my drug candidate are agonist to the 5-HT-1A receptor?
- What are the cardiac adverse effects I need to be aware of?
The new solution in 5 easy steps:
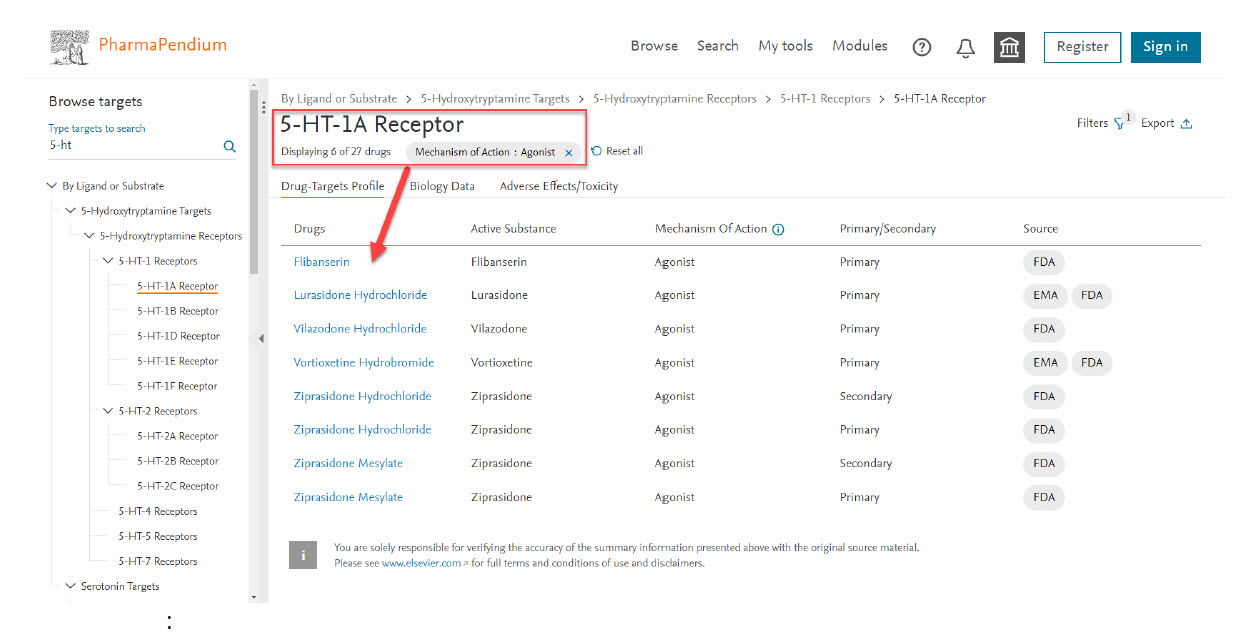
- Click on Browse Target button on the top header of PharmaPendium main page.
- Type ”5-HT-1A” in the left side filter.
Select the mechanism of action filter (top right corner) - my drug candidate is an agonist for 5-HT-1A receptor. You can also filter by drugs name, primary or secondary target and sources.
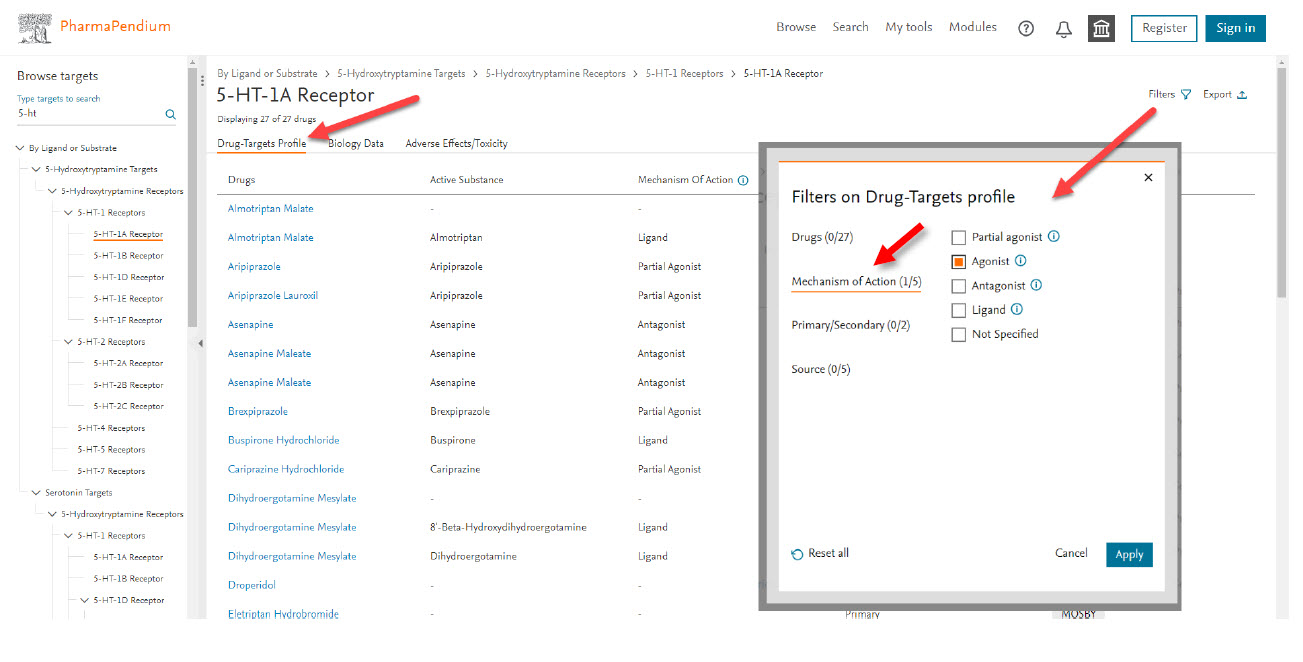
Now out of the 27, only six drugs with similar mechanism of action are displayed:
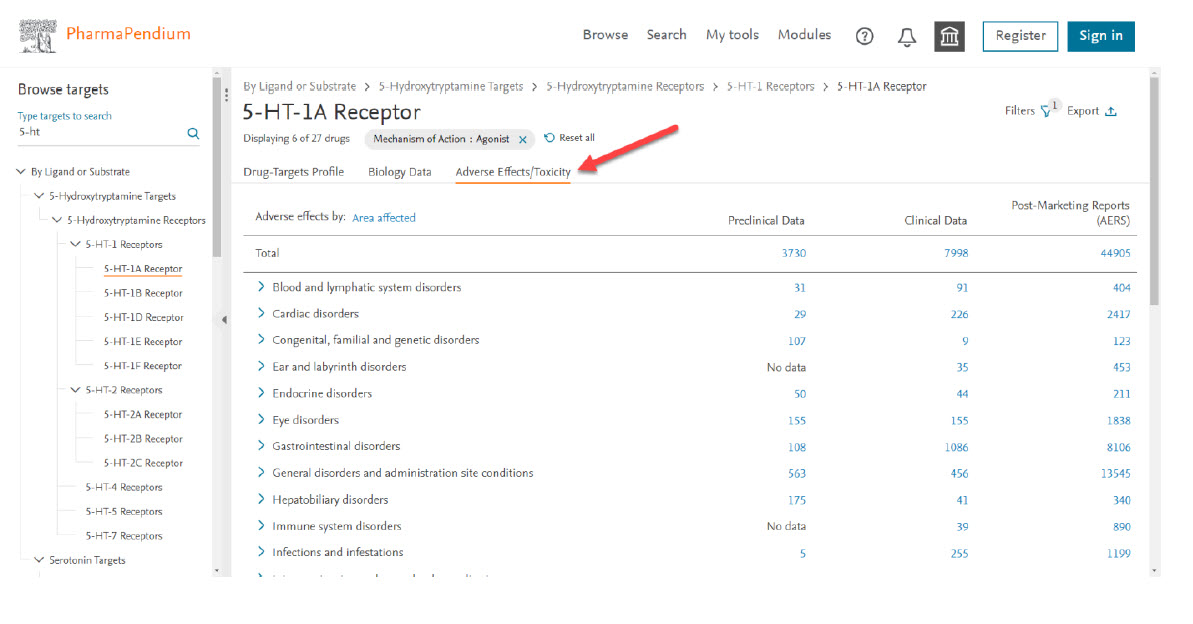
- You can now check the translational adverse effects view for these six selected drugs in the Adverse Effects/toxicity section :
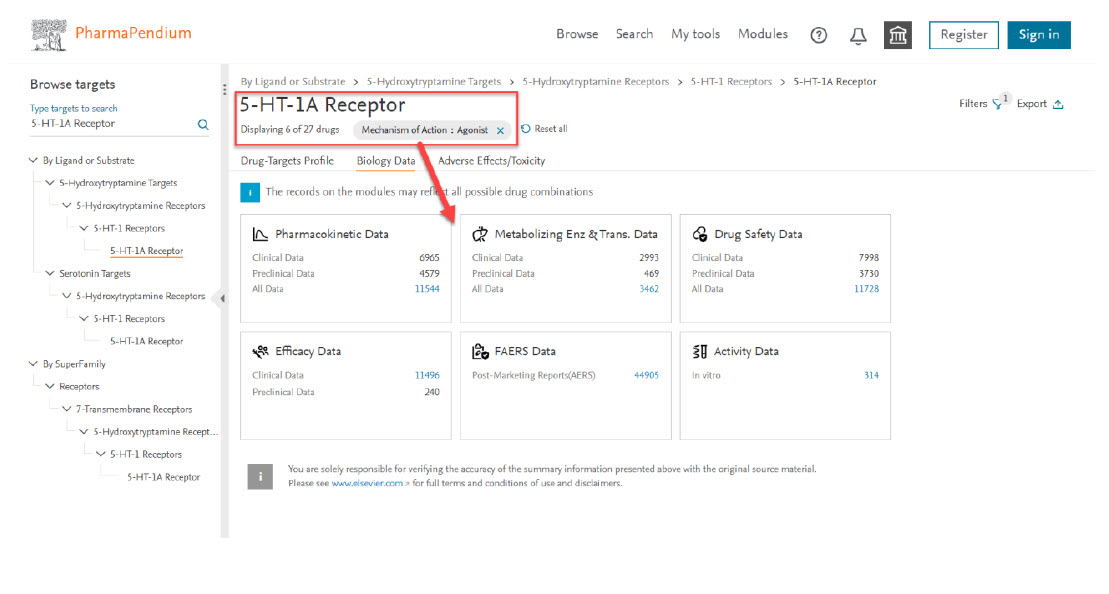
- These dynamic filters on this selection will now be applied to all modules so you can have a more accurate safety profiling assessment and focus only on these drugs of interest:
You can also export all the selected information in one go (in the top right corner)!
- The PharmaPendium Team -
Download the full release notes here.(Opens in a new tab or window)
New Meyler updated 16th edition and added content display is available in PharmaPendium.
To better support translational medicine, we have increased population characteristic, adverse effect determination and results depth for toxicity data (Safety Module) from Meyler with:
- Additional drug administration details (e.g. galenic form, concomitant drugs dose comment, therapeutic dose etc…)
- More in-depth study group details (e.g. treated disease in Disease Name, comorbidities, race/ethnicity in demographic details field, pheno- and genotype etc…)
- More details on the adverse effect/toxicity (severity fields, regulatory agency-based classifications of serious outcome)
- Details on the causal relation of the drug with the adverse effect/toxicity (the time to adverse effects, the qualitative causal relation, follow-up)
- Detailed qualitative and quantitative results (population frequencies, number of cases, qualitative results)
- Qualitative and quantitative population frequency results comparison (comparison fields with significance information)
With organized key toxicity parameters and associated data such as species, doses, route, adverse effects etc… (see below, *Release notes for December 2021).
PharmaPendium now supports the most comprehensive risk assessment and informed decision making based on in-depth population characteristics.
Do the Drug Safety tables provide new columns of information?
Yes, we added 21 fields, please see the table below:
# | Column name | Category | Before | Now |
1. | Chemistry structure | Drug | ✅ | ✅ |
2. | Study Name | Study Information | ❌ | ✅ |
3. | Adverse Effect / Toxicity | Results | ✅ | ✅ |
4. | Toxicity parameter | Results | ✅ | ✅ |
5. | Adverse Effect comment | Results | ✅ | ✅ |
6. | Severity | Results | ❌ | ✅ |
7. | Substance inducing AE | Results | ✅ | ✅ |
8. | Substance inducing AE (type) | Results | ✅ | ✅ |
9. | Severity Details | Results | ❌ | ✅ |
10. | Serious Outcome | Results | ❌ | ✅ |
11. | AE Analysis Method | Results | ❌ | ✅ |
12. | Value (number of cases) | Results | ❌ | ✅ |
13. | Value | Results | ❌ | ✅ |
14. | Qualitative Result | Results | ❌ | ✅ |
| Adverse Effect Relation to Drug | Results | ❌ | ✅ |
15. | 1. Time To AE | Results | ❌ | ✅ |
16. | 2. Qualitative Causal Relation | Results | ❌ | ✅ |
17. | 3. Follow-up | Results | ❌ | ✅ |
18. | Comparison | Results | ❌ | ✅ |
19. | Species | Study Group | ✅ | ✅ |
20. | Disease name | Study Group | ❌ | ✅ |
21. | Demographic Characteristics | Study Group | ✅ | ✅ |
22. | Development Stage | Study Group | ✅ | ✅ |
23. | Comorbidities | Study Group | ❌ | ✅ |
24. | Previous Treatment | Study Group | ❌ | ✅ |
25. | Phenotype | Study Group | ❌ | ✅ |
26. | Genotype | Study Group | ❌ | ✅ |
27. | Concomitants | Concomitants | ❌ | ✅ |
28. | Route | Drug Administration | ✅ | ✅ |
29. | Dose | Drug Administration | ✅ | ✅ |
30. | Dose comment | Drug Administration | ✅ | ✅ |
31. | Dose Type | Drug Administration | ✅ | ✅ |
32. | Treatment duration | Drug Administration | ✅ | ✅ |
33. | Therapeutic Dose | Drug Administration | ❌ | ✅ |
34. | Galenic Form | Drug Administration | ❌ | ✅ |
35. | Source | Other options | ✅ | ✅ |
36. | Year | Other options | ✅ | ✅ |
New Meyler content fields and related values can be customized in the Show/Hide columns option in the Drug Safety module:
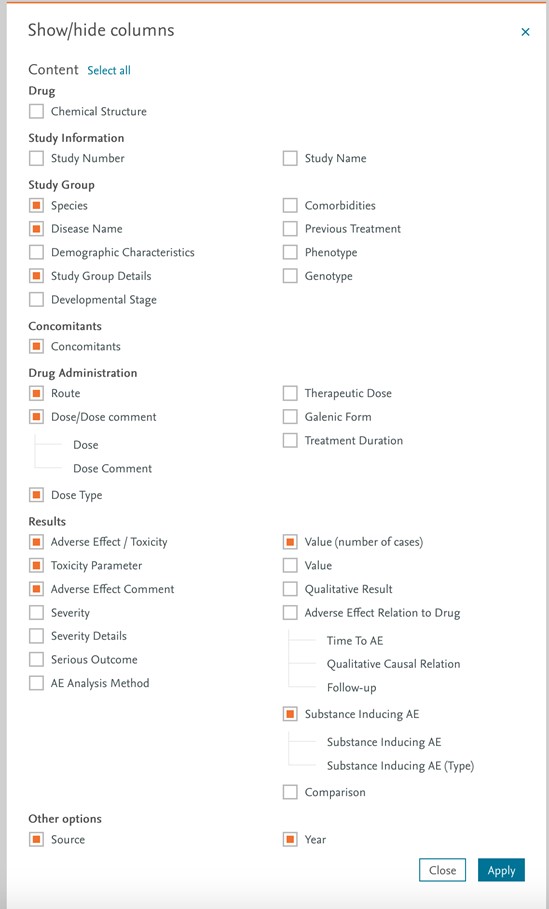
New Meyler content fields and related values can be customized in the Export feature in the Drug Safety module with similar capacities than show/hide (see above).
PharmaPendium has moved to Elsevier's new access and identity management system on Wednesday, May 04, 2022.
The new Identity Management System improves users’ sign in, registration, remote access experience, and enables Single Sign On access across Elsevier products. In addition, the new system allows users access enhanced personalized features and remote access outside institutional IP ranges.
Please note: If you are a Proxy administrator, please be aware that you must update your stanza to be compatible with the new identity management system. More information can be found at the Elsevier Access Support Center.
The new Identity Management System provides the following benefits:
- A consistent, simpler user experience for signing in and creating an Elsevier Account, including improved integration with browser password managers
- Reliable Single Sign On eliminating the need for users to sign in again as they move between multiple Elsevier products and services
- A new, integrated flow for gaining remote access from anywhere via institutional (federated) login or institutional email confirmation
- All remote access methods available and working consistently across all products
- Full compatibility with mobile devices and apps
Several Elsevier products such as Mendeley, Scopus and Embase have already adopted Elsevier’s new Identity Management System, and throughout 2022, we plan to update many of the other Elsevier products and services to bring all users of Elsevier solutions the same benefits.
Please click here for complete overview of important changes that admins and end users need to know about the new Identity Management System migration and complete list of support resources.
If you need assistance on this topic please reach out to us through any of our contact channels below.
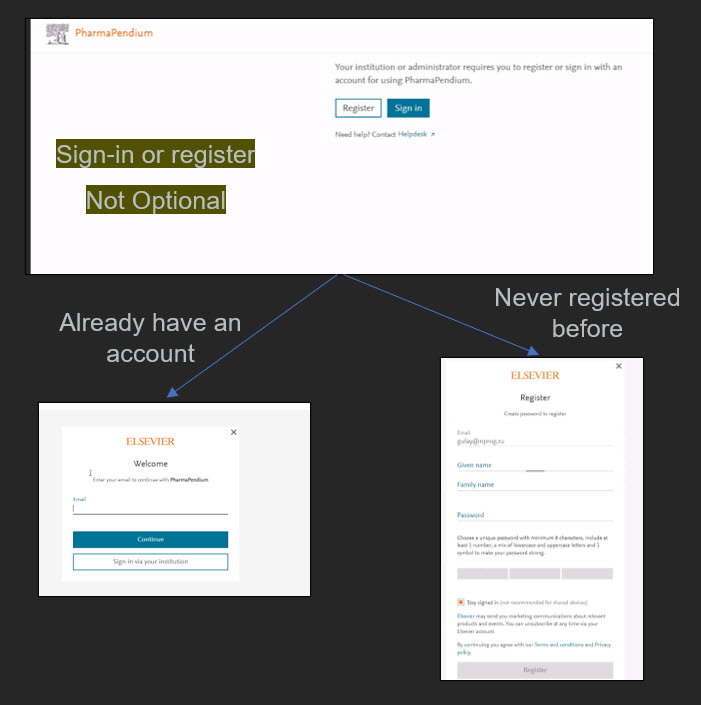
Please note: Some users will now have to register or sign-in to get access to PharmaPendium. It replaces the “one-time email request” that your administrator from your institution has asked to track usage.
This mean the user will see:
New toxicity parameters are now available in the Drug Safety Module
To improve support for determining first-in-human dosing, PharmaPendium has added over 20 toxicity parameters in the Drug Safety module.
- These values, such as NOAEL (no observed adverse effects level) and LOAEL (lowest observed adverse effect level) can be difficult to find and are not always in the drug labels.
- These parameters are being extracted from FDA and EMA approval packages from the beginning, and preclinical relevant literature from 2020.
- We have added 49000+ points of data giving PharmaPendium an unprecedented depth of safety toxicity information.
What are the benefits of this release?
- Knowing which species to test is not always obvious, so knowing the NOAEL will help you select the most sensitive specie for your pre-clinical studies. This data is also critical if regulatory agencies ask for the rationale for the specie that was used.
- With these parameters PharmaPendium will better support preclinical study designs and lead to more confident benchmarking of your drug candidate vs best-in class approved drugs in the same drug/target class.
- PharmaPendium makes it faster and easier for you to retrieve information about approved drugs and parameters such as NOAEL, AEs, AE comments, dose, route, and more.
Which toxicity parameters have been added?
Please see the table below.
Category | Toxicity Parameters | Header | Definition | Before | Now |
No adverse event | NOAEL* | No Observed Adverse Effect Level | The highest dose level of a substance that under defined conditions of exposure causes no observable/detectable adverse effect (alteration) on morphology, functional capacity, growth, development, or life span of the test animals. | ❌ | ✅ |
No adverse event | NOAEC* | No Observed Adverse Effect Concentration | The highest dose concentration of a substance that under defined conditions of exposure causes no observable/detectable adverse effect (alteration) on morphology, functional capacity, growth, development, or life span of the test animals. | ❌ | ✅ |
No adverse event | NOEL* | No Observed Effect level | The highest dose level of a substance that under defined conditions of exposure causes no effect (alteration) on morphology, functional capacity, growth, development, or life span of the test animals. | ❌ | ✅ |
Death | LD10, 20, 50*, 60, 90, 95, 99, 100 | Lethal Dose 10, 20, 50*, 60, 90, 95, 99, 100 | The dose at which 10, 20, 50*, 60, 90, 95, 99, 100% of the individuals will die. | ❌ | ✅ |
Death | LDLo | Lethal Dose Low | The lowest dosage of a compound that is introduced to the human body or that of an animal by any means apart from inhalation that will cause the death of the individual. | ❌ | ✅ |
Death | Maximum sublethal dose | Maximum sublethal dose | The maximum dose of a toxic substance that is "insufficient to cause death”. | ❌ | ✅ |
Adverse drug reaction | LOAEC | Lowest Observed Adverse Effect Concentration | The lowest concentration of a substance where the effects observed in the treated group imply an adverse effect to the subject. | ❌ | ✅ |
Adverse drug reaction | LOAEL | Lowest Observed Adverse Effect Level | The lowest dose where the effects observed in the treated group imply an adverse effect to the subject. | ❌ | ✅ |
Adverse drug reaction | LOEL | Lowest Observed Effect Level | The lowest dose where the effects observed in the treated group imply an adverse effect to the subject. | ❌ | ✅ |
Adverse drug reaction | MTD**/MTD50 | Maximum Tolerated Dose / Maximum Tolerated Dose 50 | The maximum dose that can be administered for the duration of a specific study that will not compromise the survival of the animals by causes other than carcinogenicity. | ❌ | ✅ |
Adverse drug reaction | TC50 | Toxic concentration 50 | The concentration at which toxicity occurs in 50% of cases. | ❌ | ✅ |
Adverse drug reaction | TD50 | Toxic dose 50 | The dose at which toxicity occurs in 50% of cases. | ❌ | ✅ |
Adverse drug reaction | TDlo | Toxic dose low | The lowest dose of a substance introduced by any route, other than inhalation, over any given period of time, and reported to produce any toxic effect in humans or to produce tumorigenic or reproductive effects in animals. | ❌ | ✅ |
Do the Drug Safety tables provide new columns of information?
Yes, please see the table below:
Column name | Category | Before | Now |
Chemistry structure | Drug | ✅ | ✅ |
Adverse Effect / Toxicity | Results | ✅ | ✅ |
Toxicity parameter | Results | ❌ | ✅ |
Adverse Effect comment | Results | ❌ | ✅ |
Severity | Results | ❌ | ✅ |
Substance inducing AE | Results | ❌ | ✅ |
Substance inducing AE (type) | Results | ❌ | ✅ |
Species | Study Group | ✅ | ✅ |
Demographic Characteristics | Study Group | ❌ | ✅ |
Development Stage | Study Group | ❌ | ✅ |
Route | Drug Administration | ✅ | ✅ |
Dose | Drug Administration | ✅ | ✅ |
Dose comment | Drug Administration | ❌ | ✅ |
Dose Type | Drug Administration | ✅ | ✅ |
Treatment duration | Drug Administration | ❌ | ✅ |
Source | Other options | ✅ | ✅ |
Year | Other options | ✅ | ✅ |
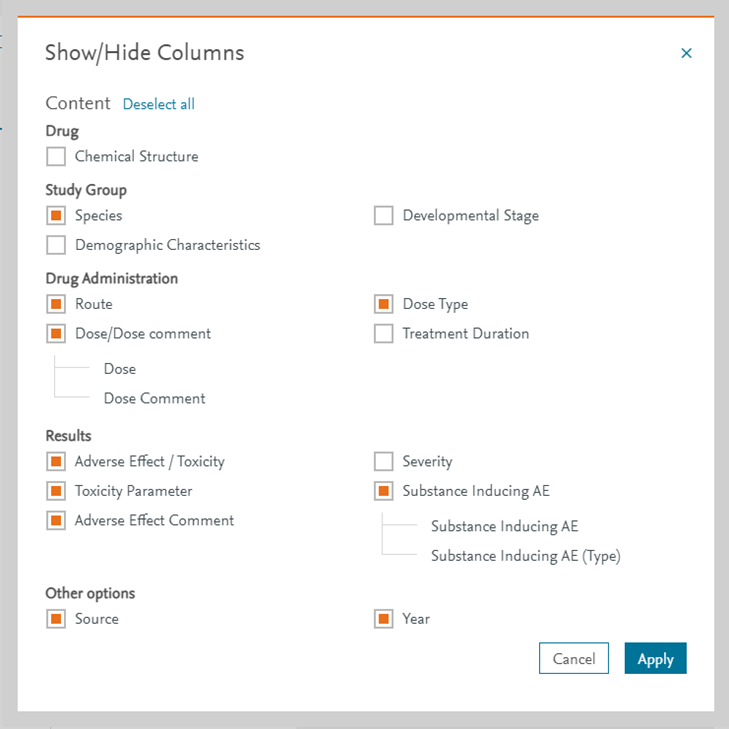
Toxicity Parameters and related values can be customized in the Show/Hide columns option in the Drug Safety module:
Toxicity Parameters and related values can be customized in the Export option in the Drug Safety module:
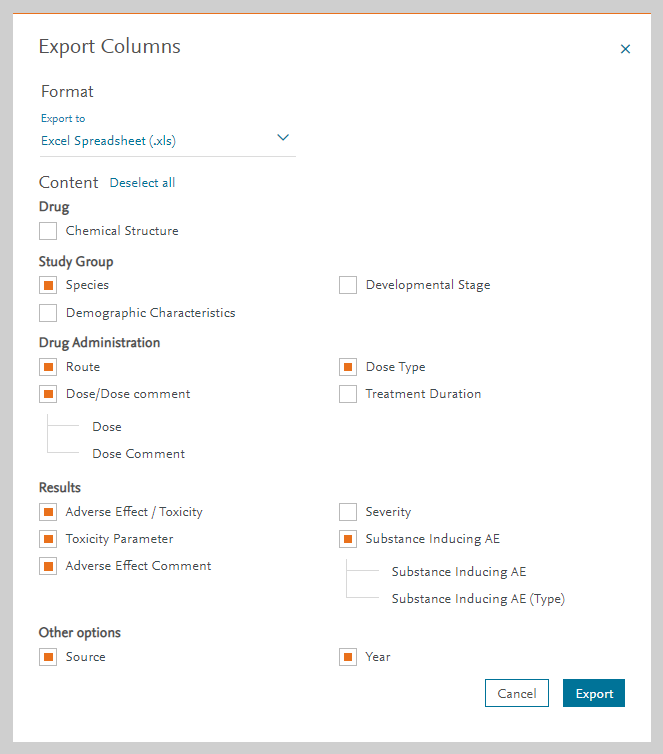
These parameters can be exported under the following formats:
- Excel Spreadsheet (.xls)
- Excel Open XML format (.xlsx)
- Tab Separated Values (.tsv)
- Comma Separated Values (.csv)
Please find below the latest release notes for the January 26, 2021 release.
PharmaPendium’s Drug-Drug Interaction Risk Calculator 2.0 (DDIRC 2.0), which is part of the PharmaPendium DMPK solution, provides a faster and more reliable method of predicting DDIs using a mechanistic approach recommended by the FDA in the last in vitro interactions studies guidance.
This calculator was developed in partnership with seven of the leading pharma companies in the world.
DDIRC 2.0 includes the following features:
- Guided data entry forms and automated data loading for easy use
- Mechanistic static model FDA compliant supporting induction and inhibition of gut and liver metabolic enzymes (CYPs and UGTs)
- Advanced visualization (Forest Plot) and filters supporting the quick identification of drug-drug interaction risk
- The calculator’s project environment to store and compare results of multiple simulations (dosing), during the drug development process
- Quick validation of results by cross-referencing with PharmaPendium’s rich set of data sources
- Ability to view the data underlying the DDI predictions for quick validation of results
DDIRC 2.0 is accessible through the PharmaPendium using a valid Elsevier account (Username & Password). Please see the instructions for DDIRC 2.0 access here.
View the detailed DDIRC2.0 User Guide for information on the approach and conventions used to make the calculations, and stepwise instructions for using the new calculator.
The release includes:
- 'Mechanism Of Action' data for over 1,500 approved drugs extracted from FDA labels and EMA annexes
- Over 4,600 extracted lines of data that are accessible through the new Target profile feature that displays when you browse for drugs or targets
- Linking of MOA for approved drugs to their primary and secondary targets
This key release will help users:
- Optimize early drug candidate selection based on DDI risk
- Prioritize clinical DDI studies and even avoid unnecessary clinical DDI trials thanks to easy access to existing data
- Make informed risk mitigation studies